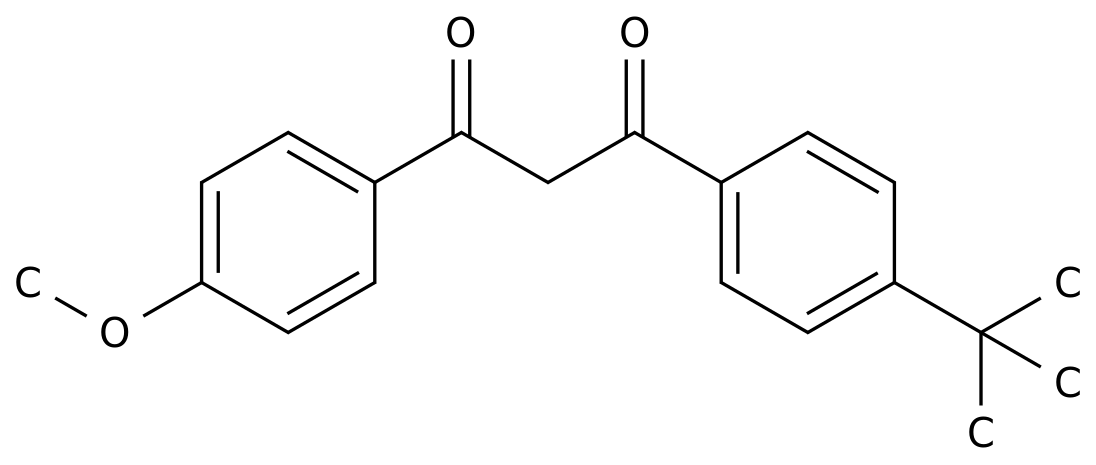
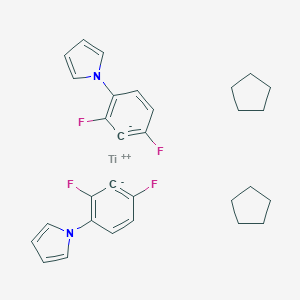
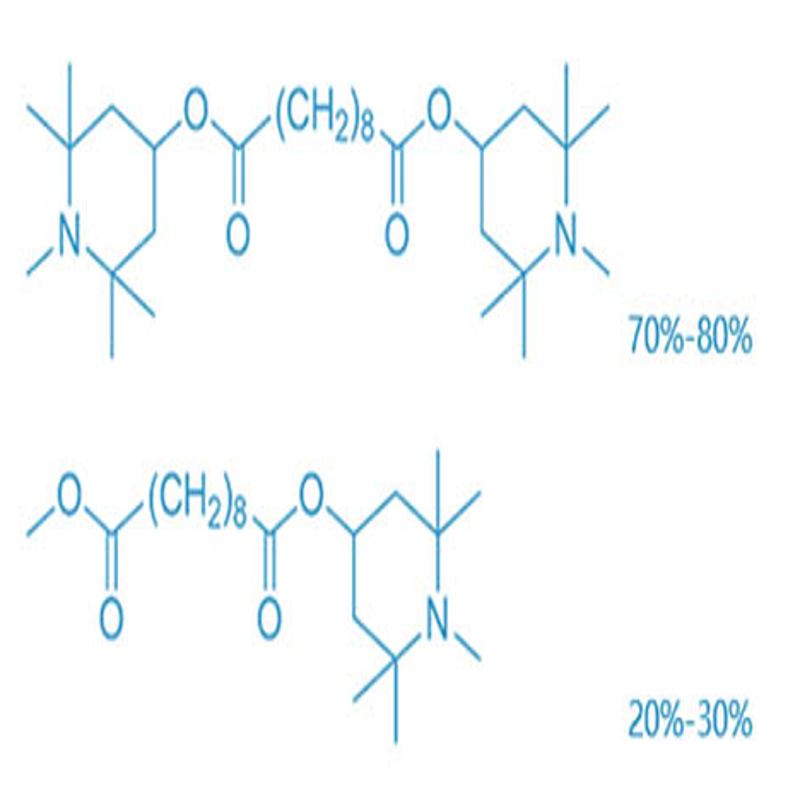
-
Categories
-
Pharmaceutical Intermediates
-
Active Pharmaceutical Ingredients
-
Food Additives
- Industrial Coatings
- Agrochemicals
- Dyes and Pigments
- Surfactant
- Flavors and Fragrances
- Chemical Reagents
- Catalyst and Auxiliary
- Natural Products
- Inorganic Chemistry
-
Organic Chemistry
-
Biochemical Engineering
- Analytical Chemistry
-
Cosmetic Ingredient
- Water Treatment Chemical
-
Pharmaceutical Intermediates
Promotion
ECHEMI Mall
Wholesale
Weekly Price
Exhibition
News
-
Trade Service
Researchers at the University of Twente in the Netherlands have found that by using nickel niobate as the anode for lithium-ion batteries, charging speed can be increased tenfold
.
In general, there are two materials that can be used as anode materials
.
First, the most common is the graphite anode, which is extremely energy-dense and can power a car for hundreds of miles without recharging
.
However, such batteries will have a process of lithium metal plating when charging, which is easy to cause fire and explosion
.
The other is a lithium titanate anode, which can be charged quickly, but has the disadvantage that its energy density decreases significantly over time, which means that the battery needs to be charged
more frequently.
Researchers at the University of Twente in the Netherlands said in a paper published in the journal Advanced Energy Materials that the new material nickel niobate can increase the charging speed while reducing the risk of
damaging the anode material, causing damage to the battery or shortening its service life.
Nickel niobate (NiNb2O6) is a new material that, in the opinion of researchers, has very attractive properties, such as the ability to return to its original level
after multiple ultrafast charge cycles.
This is mainly related to its "open and regular crystal structure", resulting in the same charge transfer channel
.
This means that it outperforms the standard anode material graphite
.
In addition, despite the complexity of manufacturing this type of nanomaterial, this is not the case with nickel niobate, since it does not require infrastructure
such as a clean room.
Moreover, nickel niobate is denser and more dense than graphite, giving it a better chance of creating lighter and simpler commercial batteries
.
Through testing, the researchers found that this version is ideal for introducing it into energy grids, electric machines that require fast charging and discharging, or electric heavy transport vehicles
.
However, if it is used in electric vehicles, further improvements
are needed.
Researchers at the University of Twente in the Netherlands have found that by using nickel niobate as the anode for lithium-ion batteries, charging speed can be increased tenfold
.
In general, there are two materials that can be used as anode materials
.
First, the most common is the graphite anode, which is extremely energy-dense and can power a car for hundreds of miles without recharging
.
However, such batteries will have a process of lithium metal plating when charging, which is easy to cause fire and explosion
.
The other is a lithium titanate anode, which can be charged quickly, but has the disadvantage that its energy density decreases significantly over time, which means that the battery needs to be charged
more frequently.
Researchers at the University of Twente in the Netherlands said in a paper published in the journal Advanced Energy Materials that the new material nickel niobate can increase the charging speed while reducing the risk of
damaging the anode material, causing damage to the battery or shortening its service life.
Nickel niobate (NiNb2O6) is a new material that, in the opinion of researchers, has very attractive properties, such as the ability to return to its original level
after multiple ultrafast charge cycles.
This is mainly related to its "open and regular crystal structure", resulting in the same charge transfer channel
.
This means that it outperforms the standard anode material graphite
.
In addition, despite the complexity of manufacturing this type of nanomaterial, this is not the case with nickel niobate, since it does not require infrastructure
such as a clean room.
Moreover, nickel niobate is denser and more dense than graphite, giving it a better chance of creating lighter and simpler commercial batteries
.
Through testing, the researchers found that this version is ideal for introducing it into energy grids, electric machines that require fast charging and discharging, or electric heavy transport vehicles
.
However, if it is used in electric vehicles, further improvements
are needed.