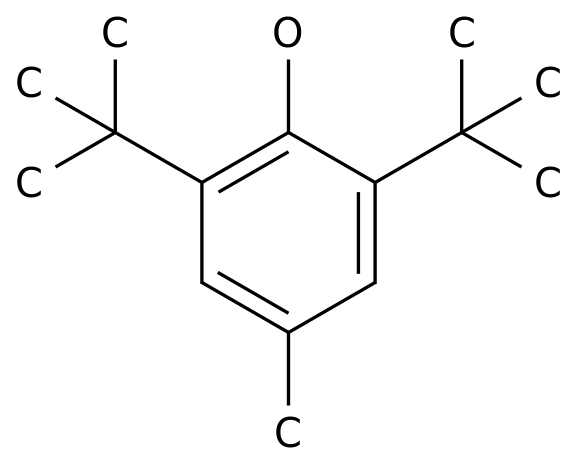
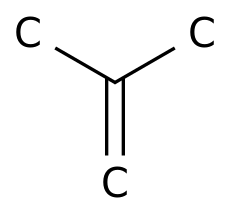
-
Categories
-
Pharmaceutical Intermediates
-
Active Pharmaceutical Ingredients
-
Food Additives
- Industrial Coatings
- Agrochemicals
- Dyes and Pigments
- Surfactant
- Flavors and Fragrances
- Chemical Reagents
- Catalyst and Auxiliary
- Natural Products
- Inorganic Chemistry
-
Organic Chemistry
-
Biochemical Engineering
- Analytical Chemistry
-
Cosmetic Ingredient
- Water Treatment Chemical
-
Pharmaceutical Intermediates
Promotion
ECHEMI Mall
Wholesale
Weekly Price
Exhibition
News
-
Trade Service
TPE is a third-generation polymer material that offers many advantages over silicone rubber or any other thermoset polymer, such as high speed, high efficiency, and economical processi.
According to a market analysis report by Mordor Intelligence, from 2018 to 2023, the global TPE market is expected to keep growing at a compound annual growth rate of 2
Meet Microbial Safety
Meet Microbial SafetyAptar Pharma's ophthalmic squeeze delivery device is the only reusable metering system approved by the FDA for preservative-free liquid medicines to da.
There are no metal parts in the drug channel of this device, so it is also suitable for very sensitive preparatio.
Several functional components of Aptar Pharma's Ophthalmic Squeeze Drug Delivery Device (OSD) are manufactured using KRAIBURG TPE's newly developed medical-grade T.
A variety of diseases can cause dry eye, such as diabetes, rheumatism, and thyroid disea.
Aptar Pharma's Ophthalmic Squeeze Delivery Device (OSD) is microbiologically safe and can be dosed simply and precisely, making it widely used as a preservative-free eye drop delivery devi.
To further optimize their products, manufacturers need a thermoplastic processable material that can come into direct contact with the drug, and that the purity, tolerance, quality, continuous availability and safety of the material used meet stringent regulatory requiremen.
KRAIBURG TPE's THERMOLAST® M compounds are tested according to relevant standards such as USP Class VI, ISO 10993-4 (hemolysis), ISO 10993-5 (cytotoxicity), ISO 10993-10 (intradermal irritation) and ISO 10993-11 (acute systemic toxicit.
Additionally, a Drug Master File (DMF) for this material is on file with the.
KRAIBURG TPE has been working with Aptar Pharma for many yea.
Passed biocompatibility testing
Passed biocompatibility testingIn the medical device market, soft-touch materials and overlays as well as hard plastics are often requir.
Trinseo offers two styrenic block copolymers (TPS) under the brands MEGOL™ TPS-SEBS compounds and RAPLAN™ TPS-SBC compounds to meet this market dema.
In 2017, Trinseo acquired API, a manufacturer of thermoplastic elastomer compounds and bioplastics, adding TPE to its line of rigid plastics produc.
Not easy to fall off
Not easy to fall offTeknor Apex recently introduced three new grades of Medalist grade medical TPE compounds for use in peristaltic pump tubing and very low temperature applications in the biopharmaceutical indust.
Materials designed specifically for peristaltic pump tubing provide the elasticity needed to keep pace with rapid pumping action and the durability needed for the tubing to retain its sha.
Compared to industrial TPEs used to replace silicone rubber, Medalist grades of medical TPE exhibit lower spalling levels, and repeated compression and expansion will not cause particles to slough off the inner and outer surfaces of the pipe, even during pump operati.
Medalist grade compounds are made with FDA-listed ingredients, certified to at least ISO 10993-5 biocompatibility standards, and are SVHC complia.
They are free of DEHP and other phthalates, BPA and lat.
Standard grades do not contain A.