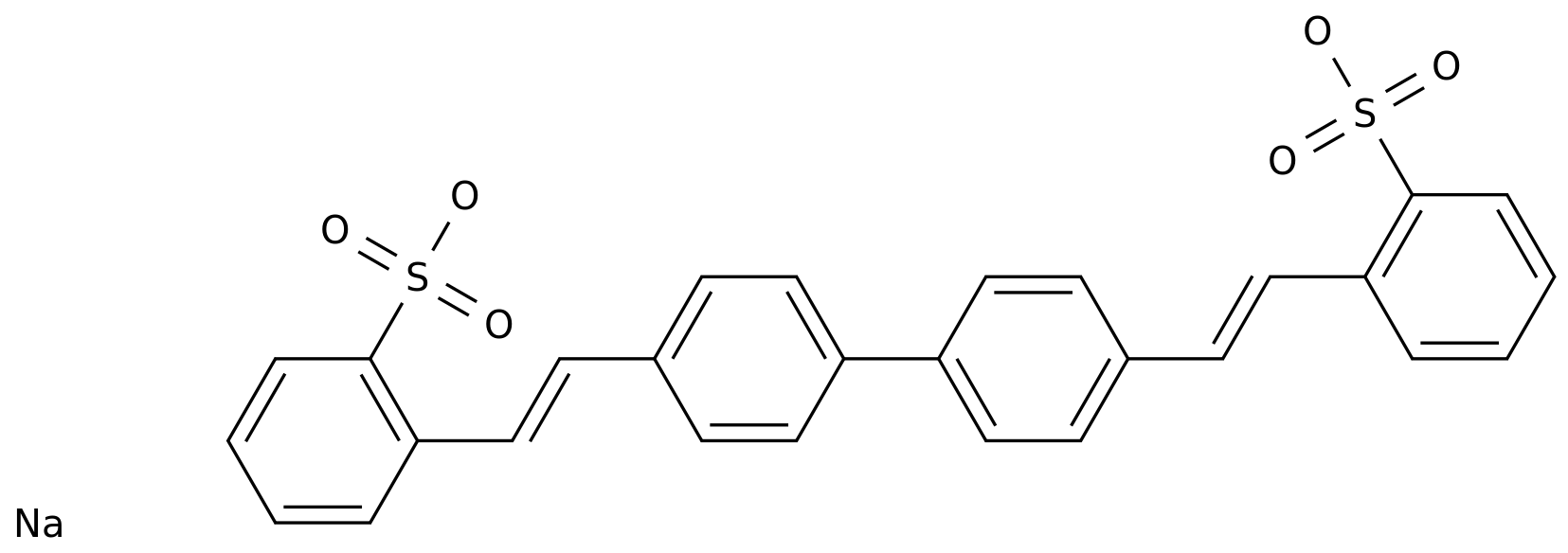
-
Categories
-
Pharmaceutical Intermediates
-
Active Pharmaceutical Ingredients
-
Food Additives
- Industrial Coatings
- Agrochemicals
- Dyes and Pigments
- Surfactant
- Flavors and Fragrances
- Chemical Reagents
- Catalyst and Auxiliary
- Natural Products
- Inorganic Chemistry
-
Organic Chemistry
-
Biochemical Engineering
- Analytical Chemistry
-
Cosmetic Ingredient
- Water Treatment Chemical
-
Pharmaceutical Intermediates
Promotion
ECHEMI Mall
Wholesale
Weekly Price
Exhibition
News
-
Trade Service
Supercapacitors using aqueous solutions as electrolytes have the advantages of low cost and high safety, and have broad application prospects in the fields of rail transit and backup power supplies
.
However, the aqueous solution is easy to solidify into ice in a low temperature environment, resulting in a sudden drop in ion conductivity, making the supercapacitor unable to work at low temperatures
.
The traditional strategy to solve this problem is to prevent the solidification of the aqueous electrolyte by adding antifreeze or using a high concentration of electrolyte
.
However, these two strategies will bring some negative effects, such as reducing ion conductivity and safety, polluting the environment and increasing costs
.
? Recently, the low-dimensional materials and chemical energy storage research group of Lanzhou Institute of Chemical Physics, Chinese Academy of Sciences has systematically studied the solidification phenomenon and electrochemical characteristics of a series of zinc salt aqueous solutions, and discovered the mechanism of the solidification aqueous solution exhibiting ultra-low ionic conductivity at low temperatures.
.
Due to the desalination characteristics of ice in the formation process, the salt will be separated from the ice, resulting in a sharp drop in the ionic conductivity of the salt-ice mixture
.
Due to the strong interaction between Zn(ClO4)2 and water molecules, the salt discharged by the ice will increase the concentration of the surrounding aqueous solution, resulting in a decrease in the freezing point of the corresponding solution
.
These concentrated solutions will form a three-dimensional network channel in the ice, which is conducive to the transmission of ions
.
At an extreme temperature of -60 ℃, Zn(ClO4)2 salt ice still exhibits an ultra-high ionic conductivity of 1.
3×10-3S cm-1
.
Using Zn(ClO4)2 salt ice as the electrolyte, the constructed zinc ion hybrid capacitor achieved 280 days of ultra-long and stable operation at low temperatures
.
Related work was published on Advanced Functional Materials with the title "SaltyIce Electrolyte with Superior Ionic Conductivity towards Low-temperature Aqueous Zinc Ion Hybrid Capacitors"
.
? The low-dimensional materials and chemical energy storage research group has been committed to the construction and basic research of high-performance low-temperature supercapacitors for many years
.
A series of progress has been made in improving the low-temperature performance of supercapacitors (SolarRRL 2018, 2, 1800223; EnergyStorage Materials, 2019, 23, 159) and widening the low-temperature voltage window of supercapacitors (Journal of Materials Chemistry A, 2020, 8, 17998)
.
The above work was supported by the National Natural Science Foundation of China, Dalian National Clean Energy Laboratory Cooperation Fund and Zhaoqing Municipal Science and Technology Bureau
.
? Low-temperature Raman scanning of Zn(ClO4)2 salt ice (a), schematic diagram of ion transport mechanism (b), and cycle stability of zinc ion hybrid capacitors (c)
.
However, the aqueous solution is easy to solidify into ice in a low temperature environment, resulting in a sudden drop in ion conductivity, making the supercapacitor unable to work at low temperatures
.
The traditional strategy to solve this problem is to prevent the solidification of the aqueous electrolyte by adding antifreeze or using a high concentration of electrolyte
.
However, these two strategies will bring some negative effects, such as reducing ion conductivity and safety, polluting the environment and increasing costs
.
? Recently, the low-dimensional materials and chemical energy storage research group of Lanzhou Institute of Chemical Physics, Chinese Academy of Sciences has systematically studied the solidification phenomenon and electrochemical characteristics of a series of zinc salt aqueous solutions, and discovered the mechanism of the solidification aqueous solution exhibiting ultra-low ionic conductivity at low temperatures.
.
Due to the desalination characteristics of ice in the formation process, the salt will be separated from the ice, resulting in a sharp drop in the ionic conductivity of the salt-ice mixture
.
Due to the strong interaction between Zn(ClO4)2 and water molecules, the salt discharged by the ice will increase the concentration of the surrounding aqueous solution, resulting in a decrease in the freezing point of the corresponding solution
.
These concentrated solutions will form a three-dimensional network channel in the ice, which is conducive to the transmission of ions
.
At an extreme temperature of -60 ℃, Zn(ClO4)2 salt ice still exhibits an ultra-high ionic conductivity of 1.
3×10-3S cm-1
.
Using Zn(ClO4)2 salt ice as the electrolyte, the constructed zinc ion hybrid capacitor achieved 280 days of ultra-long and stable operation at low temperatures
.
Related work was published on Advanced Functional Materials with the title "SaltyIce Electrolyte with Superior Ionic Conductivity towards Low-temperature Aqueous Zinc Ion Hybrid Capacitors"
.
? The low-dimensional materials and chemical energy storage research group has been committed to the construction and basic research of high-performance low-temperature supercapacitors for many years
.
A series of progress has been made in improving the low-temperature performance of supercapacitors (SolarRRL 2018, 2, 1800223; EnergyStorage Materials, 2019, 23, 159) and widening the low-temperature voltage window of supercapacitors (Journal of Materials Chemistry A, 2020, 8, 17998)
.
The above work was supported by the National Natural Science Foundation of China, Dalian National Clean Energy Laboratory Cooperation Fund and Zhaoqing Municipal Science and Technology Bureau
.
? Low-temperature Raman scanning of Zn(ClO4)2 salt ice (a), schematic diagram of ion transport mechanism (b), and cycle stability of zinc ion hybrid capacitors (c)