-
Categories
-
Pharmaceutical Intermediates
-
Active Pharmaceutical Ingredients
-
Food Additives
- Industrial Coatings
- Agrochemicals
- Dyes and Pigments
- Surfactant
- Flavors and Fragrances
- Chemical Reagents
- Catalyst and Auxiliary
- Natural Products
- Inorganic Chemistry
-
Organic Chemistry
-
Biochemical Engineering
- Analytical Chemistry
-
Cosmetic Ingredient
- Water Treatment Chemical
-
Pharmaceutical Intermediates
Promotion
ECHEMI Mall
Wholesale
Weekly Price
Exhibition
News
-
Trade Service
The Extracorporeal Membrane Oxygenation Device (ECMO), also known as the "artificial lung", which has repeatedly made outstanding achievements during the fight against the new coronary pneumonia epidemic, its core component, the membrane lung, mainly uses PMP hollow fiber membranes
.
Product chain worries surfaced
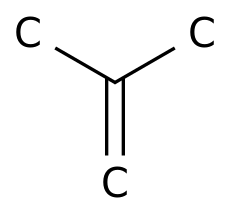
Product chain worries surfacedThe PMP film was first prepared by dimerization of propylene to obtain 4MP1 monomer
.
Secondly, propylene dimer 4MP1 is polymerized to form PMP resin under the action of "Ziegler-Natta" catalyst
.
Finally, the PMP resin will be further used in the manufacture of PMP hollow fiber membranes, which only Membrana, a subsidiary of 3M, can manufacture and supply
.
To sum up, the product chain of propylene dimer 4MP1-PMP resin-PMP hollow fiber membrane cannot be produced independently in China at present, which also restricts the localization of ECMO
.
The catalyst system is the key
The catalyst system is the keyTaking into account energy consumption and economic factors, whether the propylene dimerization process can maximize the production of 4MP1 depends mainly on the catalyst system used
.
The catalyst for the selective dimerization of propylene to produce 4MP1 is mainly based on alkali metal, at least one alkali metal element, carbonate or bicarbonate, and a co-catalyst, and the active components of the catalyst used are metal sodium and potassium
.
Dimerization of propylene using the above catalyst system is also the only way to obtain 4MP1 in industry at present
.
The new solid super alkali potassium/potassium carbonate catalyzed propylene dimerization to 4MP1, the propylene conversion rate was 43%, the selectivity of dimerization product was 95%, and the selectivity of 4MP1 was 86%
.
Because the active components of this reaction are all alkali metals, and there is little room for variation, the choice of the carrier becomes the key
.
Isomerization reaction to be suppressed
Isomerization reaction to be suppressedThe currently recognized reaction mechanism of the alkali metal system catalyzing the dimerization of propylene into 4MP1 is that propylene diffuses into the superbasic site of the catalyst, is abstracted by protons, and combines with the alkali metal on the surface of the catalyst to form an active active intermediate—allyl anion
.
Under the reaction conditions, the allyl anion is easy to generate the unstable 4MP1 anion, and the hydrogen exchange reaction between the 4MP1 anion and the propylene molecule generates a more stable allyl anion and the target product, forming a one-cycle catalytic process
.
Among them, a very small part of allyl anions will react with propylene molecules to synthesize isomer 1-hexene
.
Allyl anion has better stability than 4MP1 anion, which can control the 4MP1 anion to keep at a lower concentration during the reaction, preventing it from further polymerizing with propylene molecules to form trimers or multimers, thus ensuring dimers of high selectivity
.
In this reaction, two types of side reactions are mainly involved, one is oligomerization side reaction, and the other is isomerization side reaction
.
4MP1 is thermodynamically the most unstable hexadecene and readily isomerizes to form more stable hexadecenes
.
Therefore, in the actual production process of 4MP1, the isomerization reaction can be suppressed by reducing the content of 4MP1 in the reaction phase
.
In the industrial units that have been put into production such as the UK, the one-pass conversion rate of propylene is controlled at 7% to 11%, which is obviously to suppress the isomerization reaction
.
Another approach is to accelerate the rate of product desorption from the catalyst active sites
.
Potassium carbonate has a low specific surface area and a simple pore structure, and the alkali metal supported on potassium carbonate is an excellent catalyst for the dimerization of propylene into 4MP1, with low isomerization activity
.
The optimization of the catalyst with potassium carbonate as a carrier is to improve its pore structure, such as increasing its pore size, so that it has no effect on the isomerization activity on the basis of improving the conversion effect
.