-
Categories
-
Pharmaceutical Intermediates
-
Active Pharmaceutical Ingredients
-
Food Additives
- Industrial Coatings
- Agrochemicals
- Dyes and Pigments
- Surfactant
- Flavors and Fragrances
- Chemical Reagents
- Catalyst and Auxiliary
- Natural Products
- Inorganic Chemistry
-
Organic Chemistry
-
Biochemical Engineering
- Analytical Chemistry
-
Cosmetic Ingredient
- Water Treatment Chemical
-
Pharmaceutical Intermediates
Promotion
ECHEMI Mall
Wholesale
Weekly Price
Exhibition
News
-
Trade Service
To meet the need to create a strong post-consumer plastic feedstock, researchers at the University of Bath in the UK have developed a rapid chemical recycling process that breaks down polycarbonate within 20 minutes at room temperature
.
The process uses a zinc-based catalyst and methanol to completely decompose commercial polycarbonate particles
.
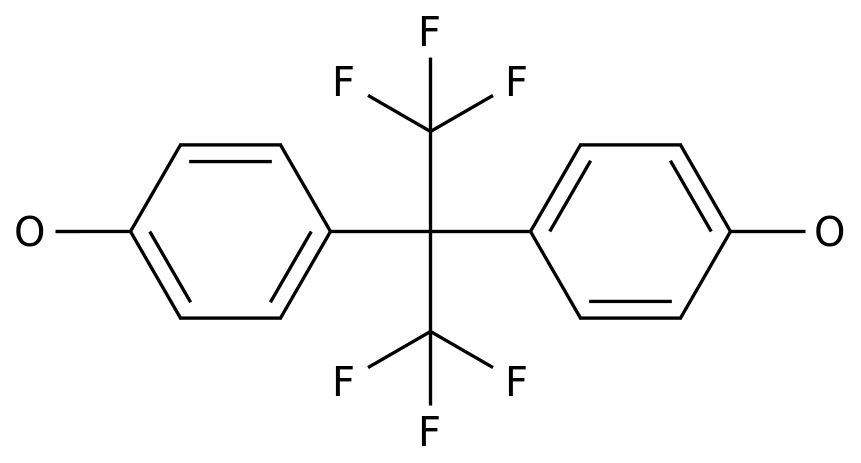
Through this process, the waste can be converted into bisphenol A (BPA) and dimethyl carbonate (DMC)
.
Polycarbonate plays an important role in architectural and engineering applications
.
The research team from the University's Centre for Sustainability and Circular Technologies (CSCT) said the catalyst used in this process could be used for BPA-PC and mixed waste from a variety of commercial sources
.
For example, at higher temperatures, PLA and PET can also be decomposed
.
According to Jack Payne, first author of a recently published paper, chemical recycling of CSCT finally enables the recovery of high-quality components
.
Harvest virgin quality resin
Harvest virgin quality resinHe explained: "After the reaction is complete, the crude BPA can be easily recovered by removing volatile components
.
Then, by recrystallization from water followed by drying, pure BPA can be obtained.
DMC should be easy It is recovered by fractional distillation of volatile components; this is necessary to separate them from the solvent 2-Me-THF, which has a comparable boiling point
.
"
“BPA and DMC separated out during this process are of virgin quality and, therefore, suitable for repolymerization
.
As fractionation is a standard industrial technique and the operating conditions are fairly mild (between 80 and 90°C), I expect large Part of the cost is related to drying the BPA to remove moisture
.
”
Practical application of chemical recycling process
Practical application of chemical recycling processThe next topic of CSCT is to expand this technology
.
According to Payne, the material recovery facility (MRF) may use the modified process
.
Payne noted, “While we have demonstrated the tolerance of our catalysts to feed impurities, including polycarbonate composites and other plastics, other ubiquitous contaminants such as food and debris have not been investigated.
.
We expect the MRF to be able to sort the high-purity feed to maintain process efficiency
.
This feed is then preferably directed to an on-site batch reactor
.
However, we have shown that under our reaction conditions, PET Still no reaction
.
So it's possible to have an input feed of polycarbonate and PET
.
After the reaction, the unreacted PET can be filtered, dried, and sent elsewhere
.
"
So how far can this process be scaled up?
Payne said: “To be economically viable, chemical recycling plants typically require 50,000 to 100,000 tons of feed per year, so we expect our process to be of considerable scale
.
However, this relies on obtaining a stable and reliable supply of polycarbonate waste.
Provenance and adequate collection and sorting infrastructure – these require substantial capital investment at the regional and national level
.
”
Payne added that assessing the cost-effectiveness of the chemical recycling process for CSCT through vis-à-vis mechanical recycling requires a comprehensive Techno-Economic Analysis (TEA) and Life Cycle Analysis (LCA)
.
Transferability is a key consideration for researchers
Transferability is a key consideration for researchers"At all stages, we were aware of the transferability from laboratory to industry," he explained.
"For catalyst design, we chose an inexpensive and abundant metal (zinc) with a simple and scalable ligand.
combined to reduce the cost of making the catalyst
.
We expect this, combined with the reduced energy intensity at ambient conditions, to make the process economically competitive with mechanical recycling
.
"
"Furthermore, if the initial upfront cost of TEA is higher compared to mechanical recycling, then our approach is able to retain material value long-term in the plastics economy, so after the payback period, it may be more economically attractive
.
"