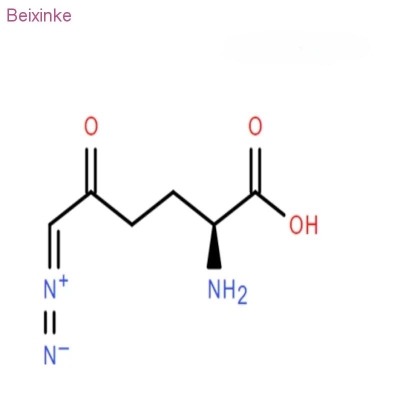
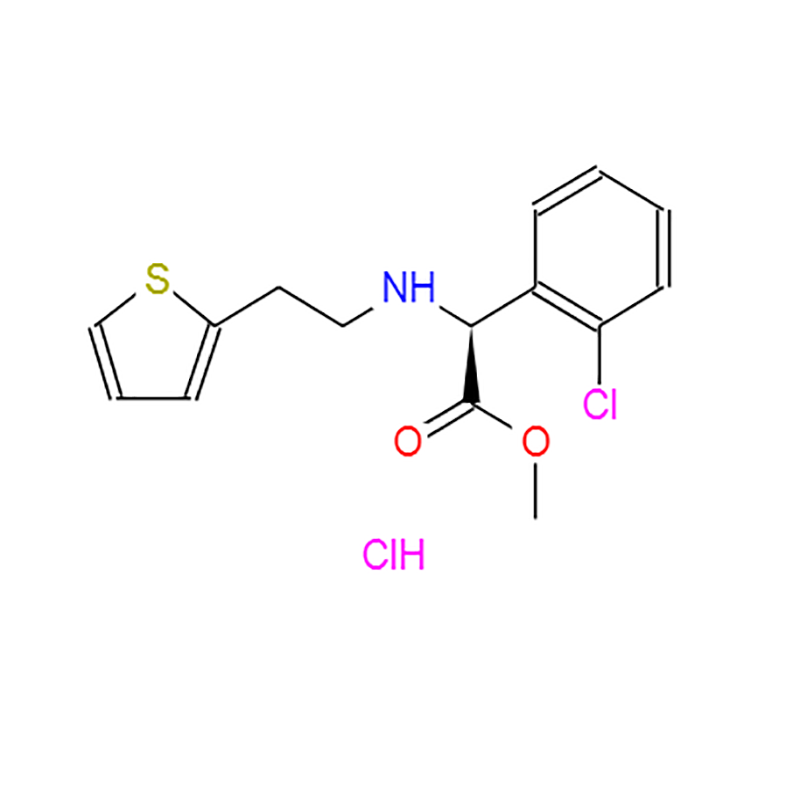
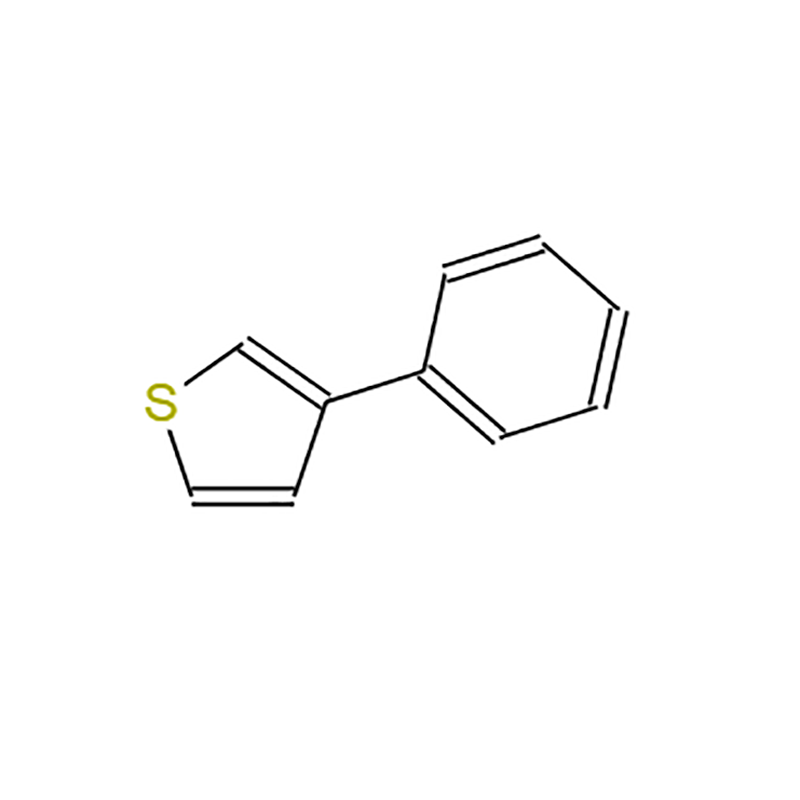
-
Categories
-
Pharmaceutical Intermediates
-
Active Pharmaceutical Ingredients
-
Food Additives
- Industrial Coatings
- Agrochemicals
- Dyes and Pigments
- Surfactant
- Flavors and Fragrances
- Chemical Reagents
- Catalyst and Auxiliary
- Natural Products
- Inorganic Chemistry
-
Organic Chemistry
-
Biochemical Engineering
- Analytical Chemistry
-
Cosmetic Ingredient
- Water Treatment Chemical
-
Pharmaceutical Intermediates
Promotion
ECHEMI Mall
Wholesale
Weekly Price
Exhibition
News
-
Trade Service
"The biopharmaceutical disposable equipment and consumables industry is developing rapidly, and the trend of localization is obvious
.
" Qin Sunxing, founder and chairman of Shanghai Lechun Biotechnology Co.
, Ltd.
, recently summarized the changes
in his industry in recent years in an interview with Zhongxin Finance.
.
" Qin Sunxing, founder and chairman of Shanghai Lechun Biotechnology Co.
, Ltd.
, recently summarized the changes
in his industry in recent years in an interview with Zhongxin Finance.
From the original foreign brands leading the way, to now local companies occupying a place, what happened in the disposable equipment and consumables industry?
Qin Sunxing bluntly said that for a long time in the past, in the process of biopharmaceutical research and development, the vast majority of enterprises used imported disposable equipment and consumables as the basis for drug development and production, but with the development of domestic disposable equipment and consumables industry, China's related products are rising
.
.
CSC Securities quoted Frost & Sullivan as saying that the global biopharmaceutical market space will increase from $286 billion in 2019 to $768 billion
in 2030.
China's biopharmaceutical market space will increase from 312 billion yuan in 2019 to 1,303 billion yuan
in 2030.
in 2030.
China's biopharmaceutical market space will increase from 312 billion yuan in 2019 to 1,303 billion yuan
in 2030.
CSC Securities Research Report believes that the new crown epidemic and geopolitical uncertainty have made biopharmaceutical companies pay more and more attention to supply chain security, bringing historic opportunities for the localization of pharmaceutical equipment consumables, and disposable technologies have set off a wave
of localization.
of localization.
In Qin Sunxing's view, local companies doing such equipment and consumables have obvious advantages
for Chinese customers.
She pointed out that local enterprises can quickly purchase according to customer needs, and pre-sales and after-sales support can achieve rapid and timely response, which is the advantage of stable supply chain and short supply cycle, and also has advantages
in terms of cost.
for Chinese customers.
She pointed out that local enterprises can quickly purchase according to customer needs, and pre-sales and after-sales support can achieve rapid and timely response, which is the advantage of stable supply chain and short supply cycle, and also has advantages
in terms of cost.
"Compared with international first-class enterprises, the overall quality of similar products of domestic enterprises still needs to be improved
.
" Qin Sunxing said frankly that the lack of key technologies and the localization fault of key links are still the main factors
restricting the localization of the whole industry chain of biopharmaceutical consumables.
.
" Qin Sunxing said frankly that the lack of key technologies and the localization fault of key links are still the main factors
restricting the localization of the whole industry chain of biopharmaceutical consumables.
Qin Sunxing said frankly that although most of the share of the domestic market in this field is still concentrated in European and American brands, local companies have begun to make efforts in research and development
.
She said that Lepure Biologics, where she works, "has several key technologies and products whose quality and performance can already be compared to international giants"
.
.
She said that Lepure Biologics, where she works, "has several key technologies and products whose quality and performance can already be compared to international giants"
.
For example, Le Pure Biotech began to research biofilms in 2018, and after long-term innovation and research, launched national chemical products from formula to process in 2021, benchmarking against the most advanced international products
.
"Only when key technologies reach the international top technical level can the international competitiveness
of China's biopharmaceutical consumables be truly enhanced.
"
.
"Only when key technologies reach the international top technical level can the international competitiveness
of China's biopharmaceutical consumables be truly enhanced.
"
Just recently, the innovation center of Lepure Biotech in its Shanghai headquarters was fully put into use
.
According to reports, the innovation center integrates R&D, verification, and application technology selection, and can provide customers with technical support and product research and development services
in the whole process and in an all-round way.
.
According to reports, the innovation center integrates R&D, verification, and application technology selection, and can provide customers with technical support and product research and development services
in the whole process and in an all-round way.
Qin Sunxing believes that putting production and manufacturing in China not only realizes localized production and ensures the security of the supply chain, but also gathers a strong talent team, so that industry-related talents can develop in the industry and play their due role
in the development of the real economy.
(End)
in the development of the real economy.
(End)