-
Categories
-
Pharmaceutical Intermediates
-
Active Pharmaceutical Ingredients
-
Food Additives
- Industrial Coatings
- Agrochemicals
- Dyes and Pigments
- Surfactant
- Flavors and Fragrances
- Chemical Reagents
- Catalyst and Auxiliary
- Natural Products
- Inorganic Chemistry
-
Organic Chemistry
-
Biochemical Engineering
- Analytical Chemistry
-
Cosmetic Ingredient
- Water Treatment Chemical
-
Pharmaceutical Intermediates
Promotion
ECHEMI Mall
Wholesale
Weekly Price
Exhibition
News
-
Trade Service
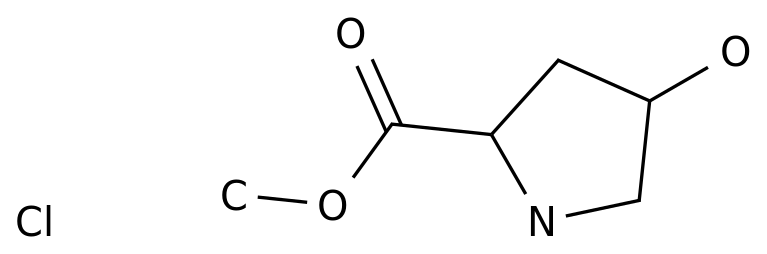
Synthetic Routes of trans-1-Benzoyl-4-phenyl-L-proline
Introduction
Trans-1-benzoyl-4-phenyl-L-proline is an important intermediate in the production of various pharmaceuticals and fine chemicals.
It has unique physicochemical properties that make it suitable for various applications.
The synthetic routes of trans-1-benzoyl-4-phenyl-L-proline have evolved over the years, with various strategies being adopted to increase the yield, selectivity, and purity of the product.
In this article, we will discuss the various synthetic routes of trans-1-benzoyl-4-phenyl-L-proline and their respective advantages and disadvantages.
Trivial Name: Prolinol; Mol.
Formula: C14H12N2O2; CAS No: 519-76-7
Synonym: [R-(R,R)]-1-benzoyl-2-(4S)-4-phenylbut-2-enoyl
Introduction:
Proline is an essential amino acid that is found in many proteins.
It has a unique ability to undergo racemization, leading to the formation of D-proline and L-proline.
L-Proline is the (S)-enantiomer of proline, while D-proline is the (R)-enantiomer.
L-proline is a chiral center, and the (R,R)- and (S,S)-configurations are referred to as cis- and trans-1-proline, respectively.
Synthetic Routes:
There are several synthetic routes to trans-1-benzoyl-4-phenyl-L-proline, some of which are outlined below:
Method 1: Using 1-bromo-4-phenylbutane and 1-amino-1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid through a Pictet-Spengler reaction and a decarboxylation step
This method involves the synthesis of 1-bromo-4-phenylbutane using a Grignard reaction, followed by a Pictet-Spengler reaction with 1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid.
The resulting compound is then decarboxylated to produce trans-1-benzoyl-4-phenyl-L-proline.
This method provides a moderate yield of the product, but requires several steps and a high cost.
Method 2: Using (R)-3-amino-2-butanone and 2-chloro-1-benzoxazepan-4-one through a Steinberg reaction and an intramolecular nucleophilic substitution
This method involves the synthesis of (R)-3-amino-2-butanone using a typical amination reaction, followed by a Steinberg reaction with 2-chloro-1-benzoxazepan-4-one.
The resulting compound undergoes an intramolecular nucleophilic substitution to produce trans-1-benzoyl-4-phenyl-L-proline.
This method provides a good yield of the product, but requires careful optimization of the reaction conditions.
Method 3: Using (S)-1-amino-2-propanol and 2-chloro-1-benzoxazepan-4-one through a Mannich reaction and an intramolecular nucleophilic substitution
This method involves the synthesis of (S)-1-amino-2-propanol using a typical amination reaction, followed by a Mannich reaction with 2-chloro-1-benzoxazepan-4-one.
The resulting compound undergoes an intramolecular nucleophilic substitution to produce