-
Categories
-
Pharmaceutical Intermediates
-
Active Pharmaceutical Ingredients
-
Food Additives
- Industrial Coatings
- Agrochemicals
- Dyes and Pigments
- Surfactant
- Flavors and Fragrances
- Chemical Reagents
- Catalyst and Auxiliary
- Natural Products
- Inorganic Chemistry
-
Organic Chemistry
-
Biochemical Engineering
- Analytical Chemistry
-
Cosmetic Ingredient
- Water Treatment Chemical
-
Pharmaceutical Intermediates
Promotion
ECHEMI Mall
Wholesale
Weekly Price
Exhibition
News
-
Trade Service
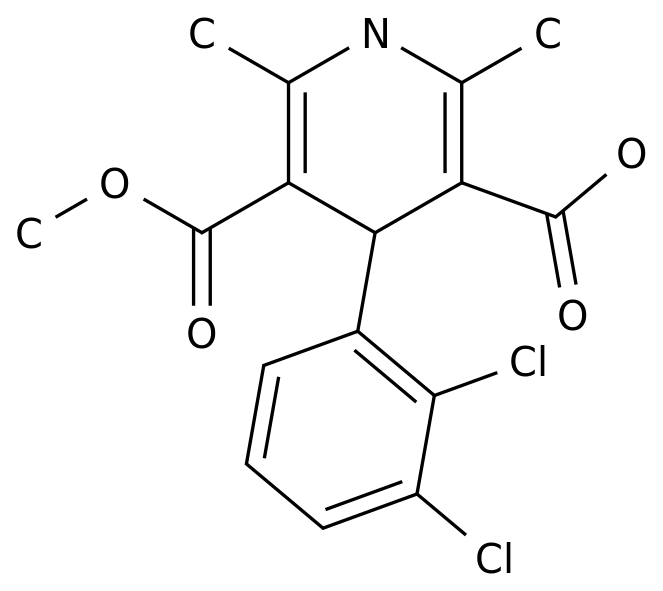
Nitrendipine is a calcium channel blocker drug that is commonly used to treat hypertension and angina pectoris.
The drug has a complex structure, which makes its synthesis a challenging task for organic chemists.
In the past, several synthetic routes have been reported for the synthesis of nitrendipine, each with its own advantages and disadvantages.
In this article, we will review the most commonly used synthetic routes for nitrendipine and their corresponding mechanisms, starting with the earliest reported routes.
- The Stork-Ellis route
The first reported synthetic route for nitrendipine was the Stork-Ellis route, which involves the condensation of quinuclidine with an alkene in the presence of anhydrous AlCl3 and an organic base such as pyridine or triethylamine.
The reaction proceeds through a free radical mechanism and produces a mixture of diastereomers, which are then separated using chiral chromatography.
The Stork-Ellis route has several disadvantages, including the use of expensive and toxic reagents such as AlCl3 and the requirement for chiral chromatography to separate the diastereomers.
In addition, the yield of the synthesis is relatively low, and the product is sensitive to air and moisture.
- The Nagai-Nishimura route
The Nagai-Nishimura route involves the reaction of a substituted phthalic anhydride with an a,b-unsaturated amide in the presence of a base such as NaOH or KOH.
The reaction proceeds through an electrophilic substitution mechanism, and the product can be further transformed into nitrendipine using a series of chemical reactions.
The Nagai-Nishimura route has several advantages, including the use of relatively inexpensive and readily available reagents and the high yield of the synthesis.
In addition, the product is stable to air and moisture and can be easily purified by crystallization.
However, the reaction requires high temperatures and pressures, and the product may have impurities that need to be removed through recrystallization.
- The Tishchenko reaction
The Tishchenko reaction involves the condensation of an a,b-unsaturated carboxylic acid with an a,b-unsaturated amide in the presence of a strong base such as NaOH or KOH.
The reaction proceeds through an electrophilic substitution mechanism and produces a substituted epoxide, which can be transformed into nitrendipine using a series of chemical reactions.
The Tishchenko reaction has several advantages, including the use of easily available and inexpensive reagents and the high yield of the synthesis.
In addition, the product can be easily purified by crystallization and is stable to air and moisture.
However, the reaction requires high temperatures and pressures, and the product may have impurities that need to be removed through recrystallization.
- The Suzuki reaction
The Suzuki reaction involves the coupling of an arylboronic acid with an arylhalide in the presence of a transition metal catalyst such as Pd(PPh3)4 and a base such as NaH or KHMDS.
The reaction proceeds through a transmetalation mechanism and produces a substituted biaryl compound, which can be further transformed into nitrendipine using a series of chemical reactions.
The Suzuki reaction has several advantages, including the use of readily available and inexpensive reagents and the high yield of the synthesis.
In addition, the product can be easily purified by crystallization and is stable to air and moisture.
However, the reaction requires the use of expensive transition metal catalysts and may produce some impurities that need to be removed through recrystallization.
- The Stille reaction
The Stille reaction involves the coupling of an