-
Categories
-
Pharmaceutical Intermediates
-
Active Pharmaceutical Ingredients
-
Food Additives
- Industrial Coatings
- Agrochemicals
- Dyes and Pigments
- Surfactant
- Flavors and Fragrances
- Chemical Reagents
- Catalyst and Auxiliary
- Natural Products
- Inorganic Chemistry
-
Organic Chemistry
-
Biochemical Engineering
- Analytical Chemistry
-
Cosmetic Ingredient
- Water Treatment Chemical
-
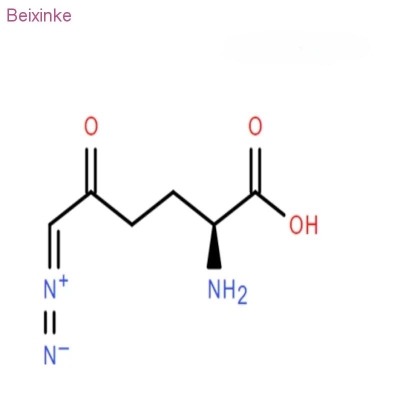
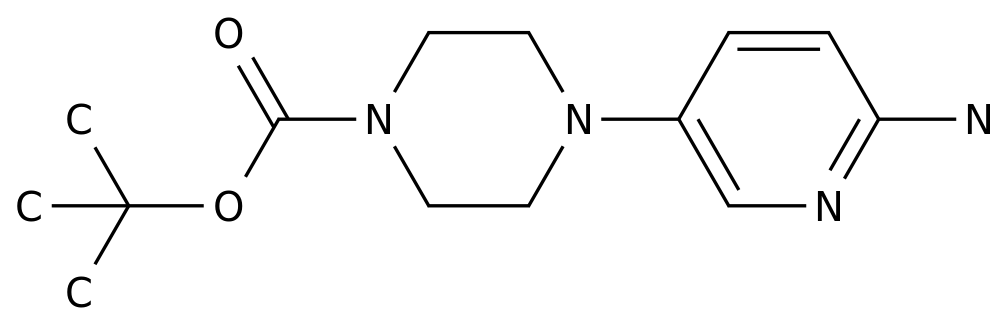
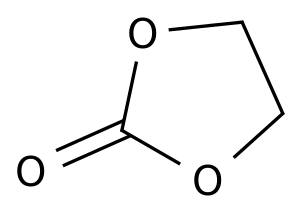
Pharmaceutical Intermediates
Promotion
ECHEMI Mall
Wholesale
Weekly Price
Exhibition
News
-
Trade Service
In recent years, as one of the three leading industries in Shanghai, the biomedical industry has developed strongly, among which the medical device industry, especially innovative medical devices
, has outstanding development.
The Shanghai Municipal Food and Drug Administration actively integrates into the overall development of the city, guards the bottom line and chases the high line, and takes multiple measures to serve the innovation and development of the medical device industry, and has achieved remarkable results
.
Since the beginning of this year, 11 products in Shanghai have entered the special review process for innovative medical devices, and 6 registration certificates have been approved, ranking among the top in the country; Some product technologies have filled the gap in related fields in China and reached the international advanced level
.
, has outstanding development.
The Shanghai Municipal Food and Drug Administration actively integrates into the overall development of the city, guards the bottom line and chases the high line, and takes multiple measures to serve the innovation and development of the medical device industry, and has achieved remarkable results
.
Since the beginning of this year, 11 products in Shanghai have entered the special review process for innovative medical devices, and 6 registration certificates have been approved, ranking among the top in the country; Some product technologies have filled the gap in related fields in China and reached the international advanced level
.
Cross-front service to tap the potential of industrial innovation
In recent years, the Shanghai Municipal Food and Drug Administration has taken the initiative to provide door-to-door services, grasp the progress of innovation and research and development of medical device enterprises through "one leader" grasp overall planning, "one network" disk resources, and "one game of chess" services, dig deep into innovation resources, timely dock the needs of enterprises to declare special review procedures for innovative medical devices, and strive to provide good services
.
.
Since 2021, the Shanghai Municipal Food and Drug Administration has promoted the excavation and promotion of the registration and listing of innovative medical devices as a "number one" project, and for the medical device field selected as a major project of independent innovation and high-tech industry development in Shanghai, the main person in charge of the bureau will lead the whole process and coordinate and promote the whole process to accelerate the product registration process
。 For example, for the domestic proton therapy system, with the strong support of the State Food and Drug Administration and the Medical Device Technical Evaluation Center of the State Food and Drug Administration (hereinafter referred to as the Device Review Center), the main person in charge of the Shanghai Municipal Food and Drug Administration coordinated the project scientific research team, clinical trial institutions and registration team, and organized special personnel to guide enterprises to do a good job in product declaration and innovative medical device special review procedures, registration testing, clinical trials, system verification, etc.
, to promote the approval of the product for marketing
in September this year 。 In addition, in response to the problems generally reported by start-up medical device enterprises such as not understanding the registration business requirements and having nowhere to consult, the main person in charge and the person in charge of the Shanghai Municipal Food and Drug Administration have strengthened communication with drug regulatory departments and clinical trial institutions in all districts of the city, conducted in-depth research on relevant enterprises, timely discovered products with innovative potential, tracked and understood the progress of research and development, and answered questions from enterprises
.
。 For example, for the domestic proton therapy system, with the strong support of the State Food and Drug Administration and the Medical Device Technical Evaluation Center of the State Food and Drug Administration (hereinafter referred to as the Device Review Center), the main person in charge of the Shanghai Municipal Food and Drug Administration coordinated the project scientific research team, clinical trial institutions and registration team, and organized special personnel to guide enterprises to do a good job in product declaration and innovative medical device special review procedures, registration testing, clinical trials, system verification, etc.
, to promote the approval of the product for marketing
in September this year 。 In addition, in response to the problems generally reported by start-up medical device enterprises such as not understanding the registration business requirements and having nowhere to consult, the main person in charge and the person in charge of the Shanghai Municipal Food and Drug Administration have strengthened communication with drug regulatory departments and clinical trial institutions in all districts of the city, conducted in-depth research on relevant enterprises, timely discovered products with innovative potential, tracked and understood the progress of research and development, and answered questions from enterprises
.
At the same time, the Shanghai Municipal Food and Drug Administration uses "one network" to revitalize resources
.
On the one hand, strengthen information exchange with relevant departments in the city, discover qualified innovative medical devices through projects such as "unveiling" innovative products, and guide enterprises to apply for special review procedures for innovative medical devices; On the other hand, relying on the Shanghai Service Station for Medical Device Innovation jointly established by the Device Review Center and the Shanghai Municipal Food and Drug Administration, we will explore the sinking of evaluation services to the forefront
of scientific and technological innovation.
In 2021, the Shanghai Municipal Food and Drug Administration, together with the Municipal Economic and Information Commission, based on Shanghai's "1+5+X" biomedical industry layout, will set up biomedical product registration guidance service workstations in 12 regions of the city, cooperate with regional market supervision departments and relevant departments, continue to explore medical device innovation resources, target advantageous products, major projects and key tracks of industrial development, and fully promote the process of R&D and industrialization
of related medical devices 。 Since 2021, more than 170 products from more than 90 enterprises in the city have been implemented docking guidance; Since the beginning of this year, 11 products have entered the special review process for innovative medical devices, of which 2 have been recommended
by the relevant district registration guidance service stations.
.
On the one hand, strengthen information exchange with relevant departments in the city, discover qualified innovative medical devices through projects such as "unveiling" innovative products, and guide enterprises to apply for special review procedures for innovative medical devices; On the other hand, relying on the Shanghai Service Station for Medical Device Innovation jointly established by the Device Review Center and the Shanghai Municipal Food and Drug Administration, we will explore the sinking of evaluation services to the forefront
of scientific and technological innovation.
In 2021, the Shanghai Municipal Food and Drug Administration, together with the Municipal Economic and Information Commission, based on Shanghai's "1+5+X" biomedical industry layout, will set up biomedical product registration guidance service workstations in 12 regions of the city, cooperate with regional market supervision departments and relevant departments, continue to explore medical device innovation resources, target advantageous products, major projects and key tracks of industrial development, and fully promote the process of R&D and industrialization
of related medical devices 。 Since 2021, more than 170 products from more than 90 enterprises in the city have been implemented docking guidance; Since the beginning of this year, 11 products have entered the special review process for innovative medical devices, of which 2 have been recommended
by the relevant district registration guidance service stations.
In addition, the Shanghai Municipal Food and Drug Administration also strives to improve the service level, implement "one-to-one" services for enterprises willing to declare innovative medical devices, guide enterprises to comprehensively sort out the core patents, technical characteristics and clinical advantages of the declared products, and strive to improve the accuracy of
the application work.
For products that fail to enter the special review procedure for innovative medical devices, help enterprises analyze the reasons, guide them to supplement and improve relevant evidence, and improve the pass rate
of the new round of application.
According to the product situation, the Shanghai Municipal Food and Drug Administration timely organizes forces, coordinates the "consultation" of technical departments such as registration, testing and evaluation, and provides technical and policy guidance
for enterprises to declare innovative medical device products.
the application work.
For products that fail to enter the special review procedure for innovative medical devices, help enterprises analyze the reasons, guide them to supplement and improve relevant evidence, and improve the pass rate
of the new round of application.
According to the product situation, the Shanghai Municipal Food and Drug Administration timely organizes forces, coordinates the "consultation" of technical departments such as registration, testing and evaluation, and provides technical and policy guidance
for enterprises to declare innovative medical device products.
Improve quality and efficiency, open up the "last mile" of registration
For enterprises whose products enter the special review process for innovative medical devices, the Shanghai Municipal Food and Drug Administration explores the establishment of a long-term service mechanism, continuously tracks and promotes the registration process, and opens up the "last mile"
of product launch.
of product launch.
The Shanghai Municipal Food and Drug Administration has explored and established a docking mechanism, organized a special team, investigated and visited enterprises whose products have entered the special review process for innovative medical devices, and tracked the progress of clinical trials and the preparation of registration materials; Go deep into medical institutions, carry out clinical trial supervision and random inspection of innovative medical devices, timely feedback the inspection situation, and do a good job in process guidance; Strengthen communication with the Device Review Center and the Yangtze River Delta Branch Center for Medical Device Technical Review and Inspection of the State Food and Drug Administration (hereinafter referred to as the Yangtze River Delta Branch Center), promote the formation of a pre-registration guidance service mechanism of coordination and liaison between the Municipal Food and Drug Administration, regular communication with designated "counselors" designated by the Yangtze River Delta Branch Center, and special guidance from the Device Review Center, and do a good job in the whole process of innovative product registration guidance services, helping enterprises solve most problems before
registration application.
registration application.
In order to optimize the registration and testing services, the Shanghai Municipal Food and Drug Administration strives to give full play to the role of the Municipal Medical Device Testing and Research Institute as the National Technical Committee for Standardization of Medical Electrical Appliances and the Key Laboratory of Medical Electrical Equipment and the Key Laboratory of Respiratory Anesthesia Equipment of the State Food and Drug Administration
。 The bureau has also opened a green channel for the registration and testing of innovative medical devices, implemented special personnel acceptance, coordinated business departments and testing departments, strived to solve the technical testing difficulties of innovative products, optimized the report approval process, and accelerated the inspection process
.
。 The bureau has also opened a green channel for the registration and testing of innovative medical devices, implemented special personnel acceptance, coordinated business departments and testing departments, strived to solve the technical testing difficulties of innovative products, optimized the report approval process, and accelerated the inspection process
.
At the same time, the Shanghai Municipal Food and Drug Administration continues to accelerate the speed of
review and approval.
In March 2021, the bureau issued the Action Plan for Medical Device Review and Approval, Improving Quality and Efficiency Expansion (2021-2022), optimizing pre-registration service guidance, promoting the implementation of file review, and expanding consultation channels; Strengthen the efficient coordination of the registration process, and explore measures
such as hierarchical and sub-channel review, on-site review, review and verification parallel.
After nearly two years of efforts, the average registration period of medical devices in Shanghai has been shortened from 165 days in 2020 to 101 days
.
Among them, the technical review was shortened from an average of 59 working days to 29 working days, and the first registration review time was shortened from an average of 93 working days to 56 working days
.
review and approval.
In March 2021, the bureau issued the Action Plan for Medical Device Review and Approval, Improving Quality and Efficiency Expansion (2021-2022), optimizing pre-registration service guidance, promoting the implementation of file review, and expanding consultation channels; Strengthen the efficient coordination of the registration process, and explore measures
such as hierarchical and sub-channel review, on-site review, review and verification parallel.
After nearly two years of efforts, the average registration period of medical devices in Shanghai has been shortened from 165 days in 2020 to 101 days
.
Among them, the technical review was shortened from an average of 59 working days to 29 working days, and the first registration review time was shortened from an average of 93 working days to 56 working days
.
In addition, the Shanghai Municipal Food and Drug Administration also actively organizes training to stimulate the momentum of industrial development
.
Since the beginning of this year, in response to the situation that medical device enterprises, especially start-up enterprises, do not understand the laws, regulations, policies and product technical review requirements of medical devices, resulting in a long time for registration and supplementation, the Shanghai Municipal Food and Drug Administration has held 16 sessions of public welfare lectures on medical device supervision to explain the legal and regulatory requirements and registration practices of medical devices, and has trained more than 1,200 medical device enterprises in total; Strengthen the capacity building of personnel in the registration guidance service workstation, organize and carry out 51 training sessions related to medical device evaluation, with more than 3,700 people participating in the training, and carry out more than 50 on-site verification and teaching; Guide industry associations (research associations) and other third-party institutions to carry out 5 training sessions on enterprise registration business, with more than 1,300 people participating in the training; Through the "Shanghai Instrument Review" WeChat public account, 166 Q&A articles were pushed, with more than 220,000 views
.
.
Since the beginning of this year, in response to the situation that medical device enterprises, especially start-up enterprises, do not understand the laws, regulations, policies and product technical review requirements of medical devices, resulting in a long time for registration and supplementation, the Shanghai Municipal Food and Drug Administration has held 16 sessions of public welfare lectures on medical device supervision to explain the legal and regulatory requirements and registration practices of medical devices, and has trained more than 1,200 medical device enterprises in total; Strengthen the capacity building of personnel in the registration guidance service workstation, organize and carry out 51 training sessions related to medical device evaluation, with more than 3,700 people participating in the training, and carry out more than 50 on-site verification and teaching; Guide industry associations (research associations) and other third-party institutions to carry out 5 training sessions on enterprise registration business, with more than 1,300 people participating in the training; Through the "Shanghai Instrument Review" WeChat public account, 166 Q&A articles were pushed, with more than 220,000 views
.
Strictly abide by the bottom line to ensure post-market quality and safety
In view of the characteristics of innovative medical devices, the Shanghai Municipal Food and Drug Administration grasps supervision and service with one hand, dynamically tracks risks, improves work systems, optimizes regulatory means, explores the establishment of long-term mechanisms for risk prevention and control, forms a closed loop of supervision, and continuously improves regulatory efficiency
.
.
The Shanghai Municipal Food and Drug Administration has formulated precise regulatory measures to ensure the quality and safety
of products after they are marketed.
For example, formulate a supervision plan for the characteristics of the first domestic proton therapy system and increase supervision and inspection; In view of the common problems existing in the supervision of post-marketing medical devices, establish a regular work meeting and risk consultation and judgment system, focusing on the risks in the performance and production quality management of innovative medical devices, and the registration, supervision, audit and other departments will jointly carry out risk consultations on system inspection, inspection and testing, adverse event monitoring and other links, put forward risk management and control suggestions, and eliminate potential risks in a timely manner
.
of products after they are marketed.
For example, formulate a supervision plan for the characteristics of the first domestic proton therapy system and increase supervision and inspection; In view of the common problems existing in the supervision of post-marketing medical devices, establish a regular work meeting and risk consultation and judgment system, focusing on the risks in the performance and production quality management of innovative medical devices, and the registration, supervision, audit and other departments will jointly carry out risk consultations on system inspection, inspection and testing, adverse event monitoring and other links, put forward risk management and control suggestions, and eliminate potential risks in a timely manner
.
In order to accurately track product risks, the Shanghai Municipal Food and Drug Administration also organized the sorting out of the conditional approval content of innovative medical device products, and a special person connected with the enterprise to understand the follow-up work, track the long-term safety and effectiveness of the product, and focus on data collection and reporting in post-marketing clinical research, follow-up visits, adverse event monitoring
, etc.
At the same time, the bureau also strengthens the flight inspection of innovative medical device manufacturers, urges enterprises to rectify the defects in the quality management system found, and does a good job of re-inspection, forming a closed loop of supervision
.
, etc.
At the same time, the bureau also strengthens the flight inspection of innovative medical device manufacturers, urges enterprises to rectify the defects in the quality management system found, and does a good job of re-inspection, forming a closed loop of supervision
.
In addition to doing a good job in daily supervision, the Shanghai Municipal Food and Drug Administration also actively uses social forces to explore the introduction of international third-party audit agencies to carry out the assessment of the quality management system of innovative medical device enterprises, help enterprises timely discover defects and systemic risks in the production quality management system, supervise and guide enterprises to rectify, and continuously improve the level of
quality management.
quality management.
The Shanghai Municipal Food and Drug Administration will continue to uphold the concept of "innovation is to grasp development, and innovation is to seek the future", implement the requirements of the reform of the medical device review and approval system, give full play to the advantages of innovation resources in Shanghai's biomedical industry, improve the working mechanism, promote the innovation and development of the medical device industry, and ensure the safety of
the masses.
the masses.