-
Categories
-
Pharmaceutical Intermediates
-
Active Pharmaceutical Ingredients
-
Food Additives
- Industrial Coatings
- Agrochemicals
- Dyes and Pigments
- Surfactant
- Flavors and Fragrances
- Chemical Reagents
- Catalyst and Auxiliary
- Natural Products
- Inorganic Chemistry
-
Organic Chemistry
-
Biochemical Engineering
- Analytical Chemistry
-
Cosmetic Ingredient
- Water Treatment Chemical
-
Pharmaceutical Intermediates
Promotion
ECHEMI Mall
Wholesale
Weekly Price
Exhibition
News
-
Trade Service
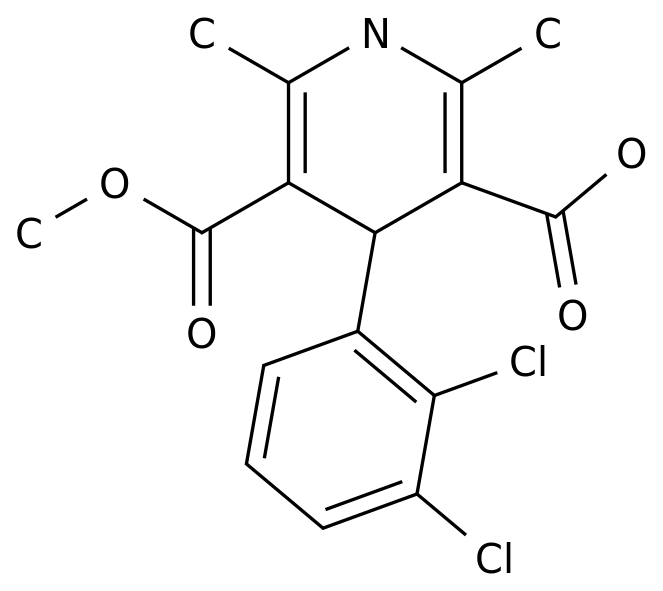
Amlodipine is a widely used calcium channel blocker medication that is primarily used to treat hypertension and angina pectoris.
The production process of amlodipine involves several steps, including the synthesis of the basic compound, its purification, and the formation of the final product.
The synthesis of amlodipine involves a multi-step process that requires the use of various chemicals and equipment.
The basic compound, which is a dihydropyridine derivative, is synthesized through a series of chemical reactions that involve the use of reagents such as halogenated compounds, nitro compounds, and amides.
The reactions are carried out in an organic solvent such as ethyl acetate or methylene chloride, and the product is purified through a series of chromatography techniques, such as column chromatography or high-performance liquid chromatography (HPLC).
Once the basic compound has been synthesized, it is purified through several steps to remove any impurities or unwanted chemicals.
The purification process typically involves the use of recrystallization, where the compound is dissolved in a solvent such as ethanol or water, and then allowed to cool and recrystallize into a pure form.
Additionally, the compound may be purified through the use of silica gel or alumina, which are used to remove impurities through adsorption.
Once the basic compound has been purified, it is then converted into the final product, amlodipine, through a series of chemical reactions that involve the use of reagents such as dilute hydrochloric acid or sodium hydrogen carbonate.
The product is then purified through HPLC or column chromatography to remove any impurities.
The production process of amlodipine also involves the use of various chemicals and equipment, including reactors, distillation columns, and chromatography columns.
These pieces of equipment are used to carry out the various chemical reactions and purification steps involved in the production of the drug.
Additionally, the production process also requires the use of various solvents and reagents, which are used to carry out the chemical reactions and purification steps.
The production process of amlodipine is a complex and multi-step process that requires the use of various chemicals, equipment, and purification techniques.
The process involves the synthesis of the basic compound, its purification, and the formation of the final product.
The final product is a pure form of the drug that is used to treat hypertension and angina pectoris.
Overall, the production process of amlodipine is a critical component of the pharmaceutical industry, and requires the use of advanced chemical engineering and technology to produce a safe and effective drug.