-
Categories
-
Pharmaceutical Intermediates
-
Active Pharmaceutical Ingredients
-
Food Additives
- Industrial Coatings
- Agrochemicals
- Dyes and Pigments
- Surfactant
- Flavors and Fragrances
- Chemical Reagents
- Catalyst and Auxiliary
- Natural Products
- Inorganic Chemistry
-
Organic Chemistry
-
Biochemical Engineering
- Analytical Chemistry
-
Cosmetic Ingredient
- Water Treatment Chemical
-
Pharmaceutical Intermediates
Promotion
ECHEMI Mall
Wholesale
Weekly Price
Exhibition
News
-
Trade Service
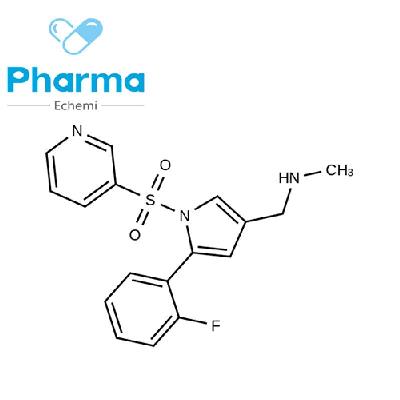
Alosetron is a drug used to treat women with irritable bowel syndrome (IBS).
The production process of alosetron involves several steps, from synthesis of the active ingredient to the final packaging of the drug.
In this article, we will take a closer look at the production process of alosetron, including the key steps involved and the equipment used in each step.
Step 1: Synthesis of the active ingredient
The first step in the production process of alosetron is the synthesis of the active ingredient, which is done through a multi-step process.
The synthesis process involves mixing various chemical compounds in appropriate ratios to produce the active ingredient.
The process is carried out in a controlled environment with strict quality control measures in place to ensure the purity and potency of the active ingredient.
Step 2: Purification of the active ingredient
After the active ingredient is synthesized, it is purified to remove any impurities that may have been introduced during the synthesis process.
The purification process typically involves several steps, including crystallization, chromatography, and filtration.
The equipment used in this step includes distillation columns, liquid-liquid extractors, and filtration systems.
Step 3: Formulation of the drug
Once the active ingredient is purified, it is formulated into the final drug product.
This involves mixing the active ingredient with other ingredients such as excipients, binders, and preservatives to create a stable and effective drug formulation.
The equipment used in this step includes mixers, granulators, and tableting machines.
Step 4: Encapsulation
After the drug formulation is complete, the active ingredient is encapsulated into small, easily digestible beads or capsules.
The encapsulation process ensures that the active ingredient is protected from the acidic environment of the stomach and released slowly in the intestine, where it is most effective.
The equipment used in this step includes capsule fillers and encapsulation machines.
Step 5: Packaging
Finally, the packaged drug is ready for distribution.
The packaging process involves placing the drug into bottles or other containers, along with the necessary labeling and packaging materials.
The equipment used in this step includes bottle-filling machines and labeling machines.
In conclusion, the production process of alosetron involves several steps, each of which is carefully controlled and monitored to ensure the quality and efficacy of the final product.
From the synthesis of the active ingredient to the packaging of the final drug, every step in the process is critical to the success of the product.
The use of advanced equipment and technology, along with strict quality control measures, ensures that alosetron is produced in a safe and effective manner.