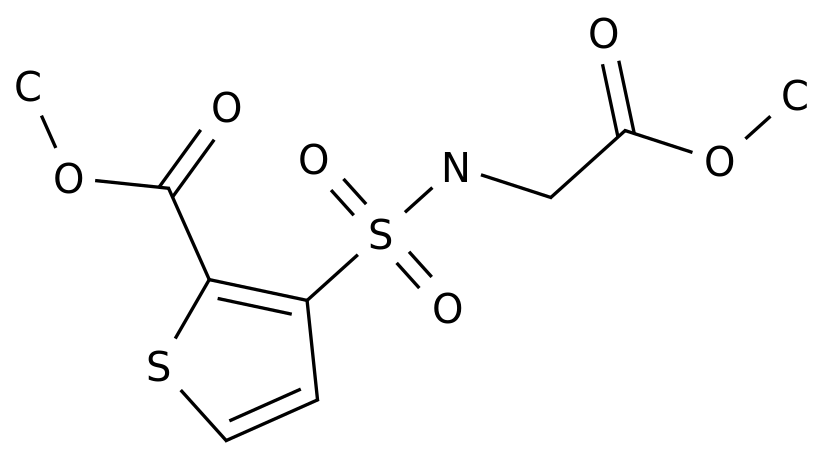
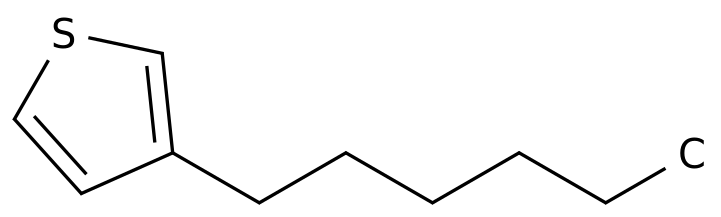
-
Categories
-
Pharmaceutical Intermediates
-
Active Pharmaceutical Ingredients
-
Food Additives
- Industrial Coatings
- Agrochemicals
- Dyes and Pigments
- Surfactant
- Flavors and Fragrances
- Chemical Reagents
- Catalyst and Auxiliary
- Natural Products
- Inorganic Chemistry
-
Organic Chemistry
-
Biochemical Engineering
- Analytical Chemistry
-
Cosmetic Ingredient
- Water Treatment Chemical
-
Pharmaceutical Intermediates
Promotion
ECHEMI Mall
Wholesale
Weekly Price
Exhibition
News
-
Trade Service
[Pharmaceutical Network Market Analysis] In order to encourage the innovation of traditional Chinese medicine and promote the development of traditional Chinese medicine, the state has issued a series of policy measures in recent years
.
For example, at the end of 2021, the National Medical Insurance Administration and the State Administration of Traditional Chinese Medicine jointly issued the "Guiding Opinions on Medical Insurance Supporting the Inheritance, Innovation and Development of Traditional Chinese Medicine", proposing "to include eligible Chinese herbal decoction pieces, proprietary Chinese medicines, and traditional Chinese medicine preparations in medical institutions into the medical insurance drug list.
"
.
For the introduction of a series of heavyweight policies, industry insiders predict that "the size of the traditional Chinese medicine market will be doubled", the development prospects of new traditional Chinese medicines will be unprecedentedly broad, and the entire traditional Chinese medicine industry will also usher in a medium and long-term golden development period
.
In fact, according to the author's understanding, benefiting from the good development prospects of the industry, Chinese medicine stocks have seen a collective rise recently.
The analysis believes that the general increase in the price of main and Chinese herbal medicines has driven the downstream prices higher, the frequent introduction of favorable policies, and the fact that traditional Chinese medicine itself has a strong consumption attributes and other factors
.
Among them, policy is the main positive factor
.
It is reported that in recent years, the approval requirements for the listing of new Chinese medicines have been gradually refined.
The effectiveness of traditional Chinese medicines is evaluated by medical theory and clinical practice experience of traditional Chinese medicine, while adhering to the orientation of clinical value
.
The "Several Policies and Measures on Accelerating the Development of Traditional Chinese Medicine Characteristics" issued in 2021 formally proposed the establishment of a "three-in-one" Chinese medicine registration review evidence system of traditional Chinese medicine theory, human experience, and clinical trials, and actively explored the establishment of real-world research evidence of traditional Chinese medicine.
system
.
In addition, in addition, some time ago, the innovation of traditional Chinese medicine welcomed heavy positive news
.
In a proposal, industry insiders suggested that the horizontal linkage of departments should be strengthened, and an effective mechanism for science and technology, medical, traditional Chinese medicine and other departments to recommend qualified new traditional Chinese medicines to enter the fast-track review and approval channel should be established
.
At present, the industry generally believes that with the continuous favorable national policies, the traditional Chinese medicine industry will gain more opportunities in 2022, and more new traditional Chinese medicine drugs will be launched at an accelerated pace
.
According to the data, the registration applications of traditional Chinese medicines have performed positively in recent years
.
Entering 2022, the number of registration applications for traditional Chinese medicine is still growing
.
According to incomplete statistics, in January 2022, CDE undertook a total of 128 new applications for registration of traditional Chinese medicines in terms of acceptance numbers, and in February CDE undertook a total of 92 new drug registration applications in terms of acceptance numbers
.
From January to February, CDE has undertaken a total of 220 new Chinese medicine registration applications
.
Analysts believe that in order to speed up the transformation and upgrading of the traditional Chinese medicine industry, in recent years, the policy warm wind has been blowing frequently, which is continuing to promote the improvement of the innovation enthusiasm of traditional Chinese medicine enterprises
.
With the continuous support of the policy, many institutions in the industry are very optimistic about the future development space of the Chinese medicine industry
.
There are also many institutions predicting that domestic innovative Chinese medicine drugs will continue to create new highs in the number of applications and approvals
.
Disclaimer: Under no circumstances does the information or opinions expressed in this article constitute investment advice to anyone
.
.
For example, at the end of 2021, the National Medical Insurance Administration and the State Administration of Traditional Chinese Medicine jointly issued the "Guiding Opinions on Medical Insurance Supporting the Inheritance, Innovation and Development of Traditional Chinese Medicine", proposing "to include eligible Chinese herbal decoction pieces, proprietary Chinese medicines, and traditional Chinese medicine preparations in medical institutions into the medical insurance drug list.
"
.
For the introduction of a series of heavyweight policies, industry insiders predict that "the size of the traditional Chinese medicine market will be doubled", the development prospects of new traditional Chinese medicines will be unprecedentedly broad, and the entire traditional Chinese medicine industry will also usher in a medium and long-term golden development period
.
In fact, according to the author's understanding, benefiting from the good development prospects of the industry, Chinese medicine stocks have seen a collective rise recently.
The analysis believes that the general increase in the price of main and Chinese herbal medicines has driven the downstream prices higher, the frequent introduction of favorable policies, and the fact that traditional Chinese medicine itself has a strong consumption attributes and other factors
.
Among them, policy is the main positive factor
.
It is reported that in recent years, the approval requirements for the listing of new Chinese medicines have been gradually refined.
The effectiveness of traditional Chinese medicines is evaluated by medical theory and clinical practice experience of traditional Chinese medicine, while adhering to the orientation of clinical value
.
The "Several Policies and Measures on Accelerating the Development of Traditional Chinese Medicine Characteristics" issued in 2021 formally proposed the establishment of a "three-in-one" Chinese medicine registration review evidence system of traditional Chinese medicine theory, human experience, and clinical trials, and actively explored the establishment of real-world research evidence of traditional Chinese medicine.
system
.
In addition, in addition, some time ago, the innovation of traditional Chinese medicine welcomed heavy positive news
.
In a proposal, industry insiders suggested that the horizontal linkage of departments should be strengthened, and an effective mechanism for science and technology, medical, traditional Chinese medicine and other departments to recommend qualified new traditional Chinese medicines to enter the fast-track review and approval channel should be established
.
At present, the industry generally believes that with the continuous favorable national policies, the traditional Chinese medicine industry will gain more opportunities in 2022, and more new traditional Chinese medicine drugs will be launched at an accelerated pace
.
According to the data, the registration applications of traditional Chinese medicines have performed positively in recent years
.
Entering 2022, the number of registration applications for traditional Chinese medicine is still growing
.
According to incomplete statistics, in January 2022, CDE undertook a total of 128 new applications for registration of traditional Chinese medicines in terms of acceptance numbers, and in February CDE undertook a total of 92 new drug registration applications in terms of acceptance numbers
.
From January to February, CDE has undertaken a total of 220 new Chinese medicine registration applications
.
Analysts believe that in order to speed up the transformation and upgrading of the traditional Chinese medicine industry, in recent years, the policy warm wind has been blowing frequently, which is continuing to promote the improvement of the innovation enthusiasm of traditional Chinese medicine enterprises
.
With the continuous support of the policy, many institutions in the industry are very optimistic about the future development space of the Chinese medicine industry
.
There are also many institutions predicting that domestic innovative Chinese medicine drugs will continue to create new highs in the number of applications and approvals
.
Disclaimer: Under no circumstances does the information or opinions expressed in this article constitute investment advice to anyone
.