-
Categories
-
Pharmaceutical Intermediates
-
Active Pharmaceutical Ingredients
-
Food Additives
- Industrial Coatings
- Agrochemicals
- Dyes and Pigments
- Surfactant
- Flavors and Fragrances
- Chemical Reagents
- Catalyst and Auxiliary
- Natural Products
- Inorganic Chemistry
-
Organic Chemistry
-
Biochemical Engineering
- Analytical Chemistry
-
Cosmetic Ingredient
- Water Treatment Chemical
-
Pharmaceutical Intermediates
Promotion
ECHEMI Mall
Wholesale
Weekly Price
Exhibition
News
-
Trade Service
The reaction mechanism, synthesis route and method of ultraviolet curable polyurethane acrylate (PUA) were reviewed, and the preparation and application of waterborne PUA, organofluorine-modified PUA, powdered PUA, hyperbranched PUA, nano-modified PUA and silicone modified PUA were mainly introduced, and the development trend
of PUA coatings was prospected.
Introduction
Radiation-curable coatings came out in the 60s of the 20th century, including ultraviolet curing (UV) and electron beam curing (EB).
In 1964, Iomont applied for the first patent for UV curable ink, and in 1968, UV curable coatings were first successfully developed by Bayer in Germany and introduced to the market coating online coatingol.
com
.
Because UV curable coatings have the advantages of fast curing rate, high efficiency, low volatile organic compound (VOC) content, green environmental protection requirements and low investment in coating equipment, their application and promotion have developed rapidly
.
It is reported that the global light-curing products market is expected to maintain a growth rate of 11.
5% in the next five years, and China will be the largest and fastest growing region
in the market.
Polyurethane acrylate (PUA) is an oligomer
that introduces double bonds through acrylateization at the end of the polyurethane molecular chain, and uses ultraviolet light to initiate a double bond crosslinking reaction under the action of a photoinitiator.
Due to the acrylic functional groups and urethane bonds in the molecule, it has the advantages of
both acrylic and polyurethane coatings.
High reactivity, excellent flexibility, low temperature resistance, strong adhesion, wear resistance, chemical resistance, weather resistance and excellent optical properties
after curing.
At the same time, PUA prepolymers with different properties can be obtained by adjusting the molecular structure and functionality to meet different needs
.
The curing film performance of ultraviolet curable polyurethane acrylate coating is good, and a small amount or even no VOC volatilizes into the atmosphere during the film formation process, which has little
environmental pollution.
However, due to its high price and oxygen inhibition polymerization, its curing rate is not as good as epoxy acrylate, so it is often used
as an auxiliary functional resin.
In most cases, the primary purpose of using PUA in formulations is to improve adhesion, reduce stress shrinkage, and increase the compliance of the cured coating
.
However, with the maturity of technology and the decline in price, PUA will also be widely used in wood, metal, plastic, optical fiber coating, ink printing and so on
.
● Synthesis of polyurethane acrylates
*Reaction mechanism of polyurethane acrylate
Isocyanates contain highly unsaturated double bonds, and their electron resonance structure is shown in
Figure 1.
As can be seen from Figure 1, the electron clouds on oxygen and nitrogen atoms have a high density and are electronegativity
.
Among them, the oxygen atom has the greatest electronegativity and is a nucleophilic center, which easily reacts with active hydrogen compounds to form hydroxyl groups, which are unstable on unsaturated carbon atoms and rearranged into urethanes or ureas
.
The electron cloud density of carbon atoms is very low, showing strong positivity, and is an electrophilic center, which is easily attacked by nucleophiles in active hydrogen compounds and nucleophilic addition polymerization reactions
.
● Synthetic route of polyurethane acrylate
Polyurethane acrylates are prepared by the reaction of polyisocyanates, hydroxyl acrylates and long-chain glycols, and since hydroxyl acrylate and polyols both contain hydroxyl groups that can react with isocyanates, there are two different protocols for synthesis:
* First expand the chain and then esterify, use excess isocyanate to react with polyol to expand the chain, synthesize polyurethane prepolymer terminated with isocyanate, and then react with hydroxyl acrylate;
* First esterification and then chain expansion, isocyanate and hydroxyl acrylate first undergo single molecule addition, and then add polyol to expand the chain to obtain PUA prepolymer.
Both routes have their own advantages and disadvantages, and in practical applications, the ideal synthetic route
can be selected according to the specific use and processing performance of the prepolymer.
Polyether-type PUA was synthesized from 2,4-toluene diisocyanate (TDI), hydroxyethyl acrylate (HEA), and polyethylene glycol (PEG) (the reaction formula is shown in Figure 2).
The results show that during the synthesis of PUA, with the increase of reaction temperature and molecular mass of prepolymer, the flexibility of the cured film is improved, and the solvent and catalyst content has little
effect on its mechanical properties.
By changing the feeding sequence of hydroxyethyl acrylate (HEA) and polyester polyols, a reasonable synthesis route
was selected by comparing the feasibility of reaction operation and product performance index.
The results show that the process of adding hydroxyethyl acrylate (HEA) and then polyester polyol is conducive to the mass distribution and molecular structure arrangement of molecules, so that the reaction process is easy to control
.
●Synthesis method of polyurethane acrylate
* Solution polymerization
In the synthesis of polyurethane acrylates, isocyanates and hydroxyl groups react strongly to exothermic, and the exothermic problem
of the reaction can be controlled by adding appropriate solvents.
Commonly used solvents are ethyl acetate, butyl acetate, toluene, xylene, etc
.
Using solvents, the reaction is easy to control, the system viscosity is low, and the conversion rate is high
.
However, the solvent is toxic, difficult to remove, the curing film shrinkage is high, the system curing speed is low, and the excellent performance of the prepolymer to the coating film is weakened, and it also does not meet the requirements of modern environmental protection development, and this method will be gradually replaced
.
* Ontology aggregation
Bulk polymerization refers to the reaction of only monomers and a small amount of initiator without solvents, high product purity, simple post-treatment and environmental protection
.
The disadvantage is that the viscosity of the system is large during the reaction process, the reaction heat is not easy to control, and the thermal polymerization side reaction
of unsaturated double bonds is easy to occur.
Isophorone diisocyanate (IPDI), polypropylene glycol (PPG), pentaerythritol triacrylate (PETA) were used as raw materials, PUA resin was synthesized by ontology method, and its synthesis route and reaction conditions were studied, and PUA curing film
with excellent mechanical properties and chemical resistance was obtained.
* Sol-gel polymerization
Organic/inorganic hybrid materials prepared by sol-gel technology have unique advantages, excellent film formation, optics and mechanical properties, and have become a research hotspot
in the field of composite materials.
Yang Zehui et al.
used ethyl orthosilicate (TEOS) to prepare silica sol, and used KH-570 as silane coupling agent to prepare ultraviolet curable polyurethane acrylate/epoxy acrylate/nano silica hybrid materials
.
The results show that the thermal loss and thermal decomposition temperature of the hybrid materials are nearly double
that of the pure resin system.
New UV-curable polyurethane acrylate
● Waterborne polyurethane acrylate
Waterborne UV-curable polyurethane acrylate (WPUA) replaces the reactive diluent monomer in traditional UV-curable coatings with water, solving the irritation, environmental pollution and unsafety caused
by volatile organic components.
Therefore, it has received extensive attention and rapid development
in recent years.
According to different emulsification methods, it can be divided into external emulsification type and self-emulsifying type, and the external emulsification type is the use of external emulsifier to disperse UV-cured PUV resin in water
under the action of high shear force.
The self-emulsifying type is to introduce hydrophilic groups on the hydrophobic polyurethane backbone, and then disperse in water, and the self-emulsifying type can be divided into anionic type, cationic type and non-ionic type
according to the introduction of hydrophilic groups.
PUA polymers are synthesized by a three-step process using toluene diisocyanate (TDI), polyethylene glycol (PEG), dihydroxymethylpropionic acid (DMPA), triethylamine (TEA) and hydroxyethyl methacrylate (HEMA) as raw materials (as shown in Figure 3), with water as a chain extender and dispersant
during the mixing of modified prepolymers.
Polyurethane acrylic oligomers
were prepared by in-situ anionic self-emulsification of isophorone diisocyanate (IP-DI), polyether polyol (NJ-210), dihydroxymethylpropionic acid (DMPA) and hydroxyethyl methacrylate (HEMA).
The effects of
reactive diluent BA/TPGDA and photoinitiator Darocur 1173 on UV curing time and film properties were studied.
By detecting the mechanical properties, solvent resistance and gel content of UV-PUA film, UV-PUA film has the best solvent resistance
when the ratio of BA and TPGDA is 5:5.
In addition, with the increase of the ratio of BA and TPGDA, the surface drying time increases
.
When the content of Darocur 1173 is 4%, the gel content reaches a maximum and the surface drying time reaches a minimum
.
Using the photopolymerization and surface activity properties of vinylbenzenesulfon, the reaction of acrylic acid with pentaerythritol was catalyzed to synthesize pentaerythritol acrylate mixtures
with different hydroxyl content.
It is further reacted with a polyurethane prepolymer prepared from isophorone diisocyanate and polyethylene glycol to obtain polyurethane acrylate
.
Studies have shown that when the catalyst dosage is 4% and the molar ratio of pentaerythritol to acrylic acid is 3.
2:1, the comprehensive performance of waterborne polyurethane acrylate UV curing film is the best
.
Difunctional waterborne polyurethane acrylate (WPUA2) and waterborne hexafunctional polyurethane acrylate (WPUA6) oligomers
were synthesized with isophorone diisocyanate (PDI), 2,2-dihydroxymethylpropionic acid (DMPA) and polyethylene glycol 400 (PEG400), hydroxyethyl acrylate (HEA), pentaerythritol triacrylate, etc.
Studies have shown that compared with WPUA2 UV curing film, the use of multifunctional WPUA6 can increase the gel rate of PUA curing film by more than 20%, reduce water absorption by 30%, lose mass by 10%, and increase the decomposition temperature by 82°C
.
While the combination of UV curing technology and waterborne coating technology brings advantages, there are also some disadvantages, such as the polymerization inhibition effect
of water on free radical polymerization.
At the same time, the removal of water requires a lot of energy, and it is difficult to cure the substrate with complex shapes, which needs to be solved
by various modification methods.
● Organofluorine modified polyurethane acrylate
Waterborne polyurethane acrylates contain a large number of active groups that give the cured film good flexibility and adhesion, but at the same time the mechanical properties, solvent resistance and water resistance are reduced
.
To overcome these shortcomings, studies have found that the introduction of fluorine segments can improve this situation
well.
Waterborne fluorinated polyurethane acrylate (WFPUA) composite emulsion was prepared
by using polyurethane (PU) emulsion, sodium ethylene ethyl acrylate (AAS) as chain extender, butyl acrylate acrylate (BA), perfluorooctylethyl acrylate (FA) and methyl methacrylate (MMA) as monomers.
The results show that the introduction of organofluorine monomer FA and the expansion of AAS effectively improve the cross-linking degree of polymers, and at the same time improve the stability of the emulsion and the water resistance of the adhesive film
.
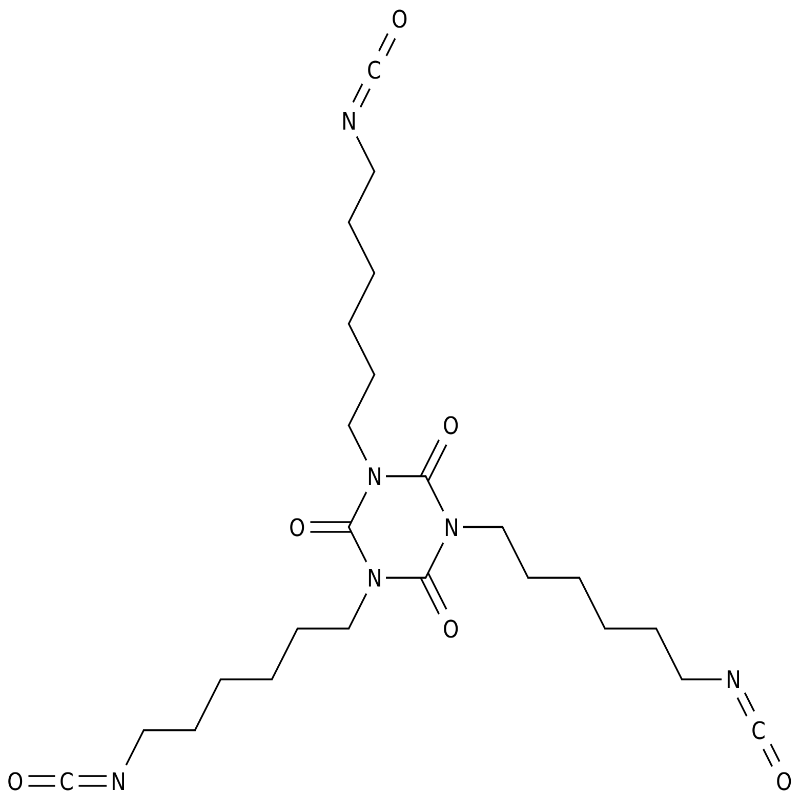
Fluorinated polyurethane acrylate (FPUA) was synthesized from hexamethylene diisocyanate (HDI)
trimer, hydroxyethyl methacrylate (HEMA), 1,6-hexanediol (HDO), 1H, 1H, 2H, 2H-trifluoro-1-octanol (TFOA).
The results show that the introduction of fluorine improves the hydrophobic antifouling performance, compatibility, mechanical properties and curing rate
of the cured film.
Polyurethane (PU) was prepared by using toluene diisocyanate (TDI), polyester diol (PE1000), trimethylolpropane (TMP), N-methyldiethanolamine (MDEA)
as raw materials.
Cationic perfluoropolyurethane acrylate polymer
was prepared by solution polymerization with butyl acrylate (BA), styrene (St), perfluoroacrylate (FA) as monomers and azobisisobutyronitrile (Al BN) as initiators.
Studies have shown that the surface of fabrics treated with cationic perfluoropolyurethane acrylate polymers has improved the contact angle between water and paraffin oil, which enhances its antifouling performance
.
● Powder polyurethane acrylate
UV-curable powder coatings combine traditional powder coatings and UV curing techniques, and their most typical feature is that the curing process is divided into two distinct stages, with the coating melt advection phase without early curing
of the resin.
Thus, it provides sufficient time for the coating to fully advect and drive away bubbles, fundamentally overcoming the shortcomings of thermosetting powder coatings, and also making up for the shortcomings
of UV-curable liquid coatings.
UV curing powder PUA coating has the advantages of no VOC emissions, high utilization rate of
raw materials, low temperature curing, and high production efficiency.
A Wenning, etc.
extruded and mixed polyurethane acrylate at 120~130 °C, cooled to the greenhouse, properly crushed, and sprayed to the suitable substrate by electrostatic electricity, and the prepared powder coating has good adhesion
.
Different concentrations of acrylate and long-chain alkyl semi-crystalline polymers DPEA-A and DPEA-B
were synthesized by modifying aromatic toluene diisocyanate (TDI), octadecyl isocyanate, hydroxyethyl acrylate (HEA) and dendritic polyether diamidide of hydroxyl end groups.
Its Tg is 45 °C and 41 °C, Tm is 123 °C and 122 °C respectively, suitable for ultraviolet curable powder coatings, and its UV curable film has excellent chemical resistance, weather resistance, scratch resistance and excellent mechanical properties
.
UV curing powder coatings have many advantages, but to achieve low temperature curing is not easy, which requires raw materials to give good storage stability to powder, powder at lower temperatures with lower melting viscosity, resin glass transition temperature, molecular weight and molecular weight distribution have specific requirements, limiting the development of
UV powder coatings.
● Hyperbranched polyurethane acrylate
The high viscosity of polyurethane acrylate oligomers is not conducive to the preparation and application of coatings, limiting their application range
.
Hyperbranched polymer is a macromolecular substance with a highly branched structure, which is one of the dendritic polymers, and the hyperbranched polymer has no linked spherical structure compared to linear polymers, which gives it lower solution viscosity and melt viscosity and higher activity and solubility
.
The substance containing NCO is prepared by reacting isoflurone diisocyanate (IP-DI) and hydroxyethyl methacrylate (HEMA), and it is synthesized with partially modified hyperbranched polyester hyperbranched polyurethane acrylic acid (HPUA) as the main resin
in the light-curing coating.
Due to the introduction of a flexible ethoxy group between the hyperbranched polyester and the acrylic end group, the steric hindrance effect between the two is reduced, and the activity of hyperbranched polyurethane acrylic acid is increased
.
Hyperbranched polyurethane acrylate (HPUA)
is synthesized by adding hydroxyl-terminated hyperbranched polyurethane (HPU-OH) and semi-additive polyurethane acrylate (IP-DI-HEA).
GPC measured the average molecular weight and polydispersity index of 7714g/mol and 1.
24, respectively, and the effects of
HPUA, epoxy acrylate and tripropylene glycol diacrylate (TPGDA) on their tensile strength and impact strength were studied.
In addition, with the addition of HPUA, the elongation at break continues to increase, and the elongation at break of EB70HPUA30 film reaches 130%.
Using glycerol as the nucleus, isophorone diisocyanate (IPDI) and diethanolamine (DEOA) were used as raw materials to synthesize a nucleated hyperbranched polyurethane containing six hydroxyl groups
.
The semi-addition product isophorone diisocyanate-hydroxyethyl acrylate (IP-DI-HEA) was modified to prepare HPUA (hyperbranched polyurethane acrylic resin) with adjustable double bond number, as shown in Figure 4, and its glass transition temperature (Tg) was 67.
8 °C, which was lower than the Tg (110.
0 °C)
of HBPUA-OH.
Hyperbranched polymers can effectively reduce the viscosity of polymers, which is conducive to the preparation and construction of coatings, but due to their complex structure, it is not conducive to accurate structural characterization and analysis, which increases the difficulty of research and limits its application
.
● Nano modified polyurethane acrylate
UV-curable nanocoatings combine UV-curable green technology with emerging nanotechnology, which makes a certain performance of coatings significantly improved
.
The NCO-terminated polyurethane prepolymer was synthesized by hexamethylene diisocyanate (HDI), N-methyldiethanolamine (MDEA), 4,4′-dihydroxybenzophenone (DH-BP) as raw materials, and then the prepolymer was grafted to the surface of nano-SiO2 to obtain nano-SiO2
with polyurethane structure.
It is added to polyurethane acrylate resin (PUA) to prepare a light-curable composite film
.
The results show that the introduction of polyurethane molecular chains on the nano-SiO2 surface effectively reduces the agglomeration phenomenon of nanoparticles in the matrix resin, improves the compatibility of the nanoparticles with the matrix resin, and improves the thermal stability and mechanical properties
of the light-curable film.
Nano silicon dioxide (SiO2) was grafted onto the surface of hexamethylene diisocyanate (HDI) trimer, and then reacted with hydroxyethyl acrylate (HEA) to prepare nano-SiO2/polyurethane acrylate (PUA) prepolymer.
The effects of
nano-SiO2/PUA prepolymer doping on the flexibility, hardness, transparency, heat resistance and impact resistance of the materials were discussed.
The results show that when the mass fraction ratio of PUA/nano SiO2 prepolymer is 60%, the comprehensive performance of the composite material is the best
.
Graphene-containing PUA nanocomposites
were obtained by composite graphene nanosheets (F-GNS) functionalized with polyurethane acrylates using 3-methacryloyloxypropyltrimethyloxysilane.
This nanocomposite increases its thermal degradation temperature by 16°C, and when the mass fraction of F-GNS reaches 1%, the energy storage modulus and glass transition temperature of the nanocomposite increase
.
This is attributed to the fact that covalent modification of graphene can improve the interaction
between the F-GNS and PUA interfaces dispersed in the polymer matrix.
Waterborne polyurethane acrylate prepolymers
containing graphene oxide were prepared by in-situ polymerization using IPDI, polyether diol, HEMA, DM-PA, GO (graphene oxide) as raw materials.
Its wear resistance, aging resistance, mechanical properties, air permeability, thermal stability are improved, and the resistivity of the cured film is greatly reduced, which can be used in conductive coatings
.
The structure and morphological characteristics
of FGO/PUA nanocomposite coatings were characterized by FTIR, XRD, and TEM by incorporating homemade functionalized graphene oxide (FGO) into polyurethane acrylate (PUA) (as shown in Figure 5) by UV curing.
The results show that the FGO sheets are uniformly dispersed in the PUA matrix and form a strong interfacial bond with PUA, which is due to the formation of a cross-linking network between FGO and PUA after UV curing, and the introduction of FGO effectively enhances the thermal stability and mechanical properties
of the host polymer.
A series of ZnO nanocomposites
with different ZnO content were prepared by UV curing system.
To ensure good dispersion of ZnO nanoparticles in the PUA matrix, ZnO nanoparticles were modified with silane coupling agents, and WAXD and SEM analysis showed that the surface-modified ZnO nanoparticles were uniformly dispersed in the
PUA matrix.
Compared with pure PUA, the hardness of the cured film increases from 0.
03GPa to 0.
056GPa, the modulus of elasticity increases from 2.
75GPa to 3.
55GPa, and the hydrophobicity and thermal stability of the composite film are improved
.
Due to the high surface activity of nanomaterials, dispersing them into the coating matrix is a key technology
for the application of nanomaterials in coatings.
The application of supercritical fluid (SCF) technology to the preparation of nanoparticles, the surface tension of supercritical fluid is almost zero, has high diffusion performance, can be fully mixed with the matrix, and maximizes its solubility ability, which has recently attracted widespread attention
.
● Silicone modified polyurethane acrylate
Silicone modified UV curing PUA has the advantages of low surface tension, high weather resistance, hydrophobicity, chemical resistance, heat resistance, etc.
, and it introduces silicone to modify oligomers during synthesis, giving the coating film good heat resistance, water resistance and mechanical properties
.
Hyperbranched polymer (HBP-OH) was synthesized by N,N-dihydroxyethyl-phthalamoylbenzoic acid and methyl N,N-dihydroxyethyl-3-aminopropanoate
monomer centered on trimethylolpropane (TMP).
On this basis, a UV-curable hyperbranched photosensitive silicone polyurethane acrylate prepolymer (HBPSUA) was synthesized using hydroxyethyl acrylate (HEA), isophorone diisocyanate (IPDI) and alkyl hydroxysilicone oil (SD9134) as raw materials, and the effects of
synthetic route and catalyst dosage on the reaction and product were discussed.
Studies have shown that while maintaining the properties of prepolymer silicones, the material has good flexibility, wear resistance, high hardness and excellent chemical resistance, and can improve the miscibility of silicones with other acrylate monomers and prepolymers
.
Wei Jun et al.
used hexamethylene diisocyanate (HDI)/toluene-2,4-diisocyanate (TDI) and polyethylene glycol (PEG) reaction to prepare polyurethane prepolymer, and the surface grafting modification
of nano-SiO2 was carried out with prepolymer.
Then it is uniformly dispersed into PUA, and the results show that the thermal stability and mechanical properties
of the hybrid coating can be significantly improved by adding modified nano-SiO2.
Different aliphatic diisocyanates, acrylic acid monomers, alcohol-terminated polysiloxanes are used as the main raw materials
.
Using the step-by-step synthesis method, the silicone modified polyurethane was first synthesized, then the hydrophilic group was introduced, and finally the acrylic monomer was introduced, and the catalyst used a mixture of dibutyltin laurate and organocarboxylic acid rare earth
.
Reflow at a certain temperature, optimize the reaction conditions, so that the prepared light-curable silicone modified waterborne polyurethane acrylate coating has good yellowing resistance, high hardness and other excellent properties
.
Photosensitive silicone-containing polyurethane acrylate prepolymer (Si-IPDI-HEA)
was synthesized by hydroxyl-terminated polysiloxane, polypropylene glycol (PPG-2000), isophorone diisocyanate (IPDI), 2-hydroxyethyl acrylate (HEA), hydroquinone (HQ).
Studies have shown that Si-IPDI-HEA has a higher polymerization rate and double bond conversion than ordinary acrylic monomer resins, while introducing siloxanes into the prepolymer improves the thermal stability of UV-cured films and reduces their surface energy
by changing the microstructure.
Organosilicon-modified polyether polyurethane acrylate oligomers (Si5E5PUA) were prepared by using hydroxyalkyl polysiloxane (Q4-3667), isophorone diisocyanate (IPDI), polypropylene glycol (PPG-2000), diethanolamine and β-hydroxyethyl acrylate (HEA) acrylate as raw materials, as shown in
Figure 6.
The results show that Si5E5PUA has good compatibility with acrylate monomer, the double bond conversion rate in the system reaches more than 90%, has excellent photopolymerization performance, and effectively improves the glass transition temperature
of the cured film.
In order to improve the heat resistance, water resistance and flexibility of polyurethane acrylic, the organosiloxane block modified polyurethane copolymer was synthesized by a two-step reaction of excess isophorone diisocyanate (IPDI), polyester diol and alkyl hydroxysilicone oil
.
The end is then capped by acrylic monomer to obtain a UV-curable silicone polyurethane acrylate prepolymer
.
The results show that when the mass fraction of hydroxypropylpolysiloxane (PDMS) is 5%~7%, the elongationat break, heat resistance and hydrophobicity of the coating film are significantly improved
.
Conclusion
With the development of polymer science, environmental protection and energy conservation have received more and more attention, the development direction and product structure of the coating industry have changed
.
The reactive diluent used in the components of UV-curable coatings still contains organic volatiles, which have certain irritation and toxicity, and water-based UV-curable coatings are a direction
for future development.
Because it effectively avoids the irritation and toxicity
of reactive diluents.
In addition, UV curable powder coating is also an important development direction, first of all, UV curable powder coating integrates the advantages of powder coating and light curing, making up for the shortcomings of general traditional powder coatings, so that it can not only be used in metal, but also in plastic and wood products and other heat-sensitive components, expanding the scope of
use of powder coatings.
Secondly, ultraviolet curable nano coatings concentrate light curing green technology and emerging nanotechnology, with the continuous improvement of science and technology, nano coatings will be widely used, which will be conducive to promoting the green development
of coatings.