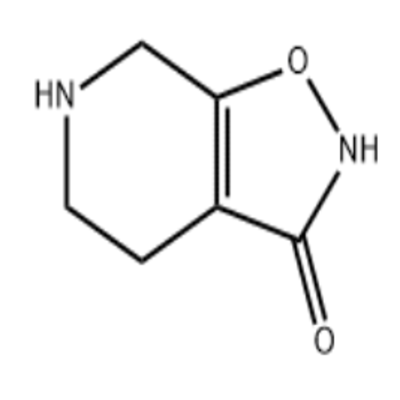
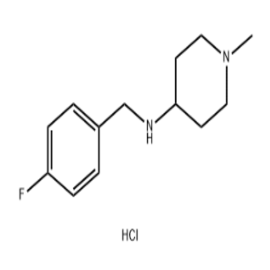
-
Categories
-
Pharmaceutical Intermediates
-
Active Pharmaceutical Ingredients
-
Food Additives
- Industrial Coatings
- Agrochemicals
- Dyes and Pigments
- Surfactant
- Flavors and Fragrances
- Chemical Reagents
- Catalyst and Auxiliary
- Natural Products
- Inorganic Chemistry
-
Organic Chemistry
-
Biochemical Engineering
- Analytical Chemistry
-
Cosmetic Ingredient
- Water Treatment Chemical
-
Pharmaceutical Intermediates
Promotion
ECHEMI Mall
Wholesale
Weekly Price
Exhibition
News
-
Trade Service
In order to standardize the management of the annual reports of drug marketing authorization holders, the State Drug Administration issued a notice today to officially release the "Regulations on the Administration of Drug Annual Reports" (hereinafter referred to as the "Administrative Regulations")
.
The "Administrative Regulations" will come into effect from the date of issuance, and the collection module of the annual drug report will be activated at the same time
.
The annual report shall be approved by the legal representative of the enterprise or the person in charge of the enterprise (or its authorised person in writing), and before April 30 each year, the report information of the previous year shall be reported through the drug annual report system
.
In view of the first implementation of the drug annual report system in China, the drug annual report collection module is still in the trial operation stage, and the deadline for filling in the 2021 annual report information is August 31, 2022
.
The purpose of implementing the drug annual report system is to supervise the implementation of the main responsibility for the whole-process quality management of drug marketing authorization holders
.
According to the requirements of the "Drug Administration Law", drug marketing license holders shall establish an annual reporting system, and report the production and sales of drugs, post-marketing research, and risk management to the drug regulatory department in accordance with regulations
.
The drug supervision and administration department may use the annual report as a reference material and a research and judgment basis for supervision and inspection, risk assessment, credit supervision and other work
.
By comparison, it is found that China's drug annual report system fully draws on the US FDA's annual report regulations, and has been revised and improved on the basis of extensive consultation with the industry in the early stage, which is more in line with China's national conditions and regulatory reality
.
Division of labor and cooperation among drug regulatory departments The "Administrative Regulations" make it clear that the State Drug Administration is responsible for guiding the management of national drug annual reports
.
The drug regulatory departments of the people's governments of provinces, autonomous regions and municipalities directly under the Central Government shall be responsible for supervising and administering the establishment and implementation of the annual report system by holders within their respective administrative regions (including domestic enterprise legal persons established by overseas holders), and provide guidance on the completion of annual reports
.
The Information Center of the State Drug Administration is responsible for the construction of the drug annual report information system and the summary statistics of relevant information, and to update the relevant information of the annual report to the corresponding drug variety files and drug safety credit files in a timely manner
.
The review, inspection, verification, monitoring and evaluation and other specialized drug technical institutions established or designated by the State Drug Administration shall inquire and use the information in the annual drug report according to their duties
.
The Marketing Authorization Holder is the Responsible Body of the Annual Report The Administrative Regulations emphasize that the Drug Marketing Authorization Holder is the responsible body of the annual report and is responsible for its authenticity and accuracy
.
If the holder is an overseas enterprise, the domestic enterprise legal person designated by it shall perform its annual reporting obligations
.
The holder shall write the annual report according to the annual report template.
In principle, one holder shall write one annual report every year
.
The information in the annual drug report shall be true, accurate, complete and traceable, and comply with the requirements of laws, regulations and relevant regulations
.
The annual report shall be approved by the legal representative of the enterprise or the person in charge of the enterprise (or its authorised person in writing), and before April 30 each year, the report information of the previous year shall be reported through the drug annual report system
.
Online filing + information sharing The drug annual report collection module is divided into the enterprise side and the regulatory side
.
The information collected by the enterprise includes two aspects: the public part and the product part
.
The public part includes information on the holder, an overview of quality management, the construction and operation of the pharmacovigilance system, acceptance of overseas commissioned processing, and acceptance of inspections by overseas regulatory agencies; the product part includes product specific information, production and sales, post-marketing research and change management, risk management,
etc.
In order to facilitate enterprises to fill in the report, the basic information of enterprises, basic product information, etc.
can be automatically brought out by the drug annual report collection module in the drug business application system; the approval document number involved in the post-marketing change management, the approval type change, and the record type change.
The record number and related information can be queried through the drug business application system (enterprise side)
.
If the holder fails to submit the annual report as required, in accordance with the provisions of Article 127 of the Drug Administration Law, it shall be ordered to make corrections within a time limit and given a warning; if it fails to make corrections within the time limit, a fine of not less than 100,000 yuan but not more than 500,000 yuan shall be imposed.
fine
.
.
The "Administrative Regulations" will come into effect from the date of issuance, and the collection module of the annual drug report will be activated at the same time
.
The annual report shall be approved by the legal representative of the enterprise or the person in charge of the enterprise (or its authorised person in writing), and before April 30 each year, the report information of the previous year shall be reported through the drug annual report system
.
In view of the first implementation of the drug annual report system in China, the drug annual report collection module is still in the trial operation stage, and the deadline for filling in the 2021 annual report information is August 31, 2022
.
The purpose of implementing the drug annual report system is to supervise the implementation of the main responsibility for the whole-process quality management of drug marketing authorization holders
.
According to the requirements of the "Drug Administration Law", drug marketing license holders shall establish an annual reporting system, and report the production and sales of drugs, post-marketing research, and risk management to the drug regulatory department in accordance with regulations
.
The drug supervision and administration department may use the annual report as a reference material and a research and judgment basis for supervision and inspection, risk assessment, credit supervision and other work
.
By comparison, it is found that China's drug annual report system fully draws on the US FDA's annual report regulations, and has been revised and improved on the basis of extensive consultation with the industry in the early stage, which is more in line with China's national conditions and regulatory reality
.
Division of labor and cooperation among drug regulatory departments The "Administrative Regulations" make it clear that the State Drug Administration is responsible for guiding the management of national drug annual reports
.
The drug regulatory departments of the people's governments of provinces, autonomous regions and municipalities directly under the Central Government shall be responsible for supervising and administering the establishment and implementation of the annual report system by holders within their respective administrative regions (including domestic enterprise legal persons established by overseas holders), and provide guidance on the completion of annual reports
.
The Information Center of the State Drug Administration is responsible for the construction of the drug annual report information system and the summary statistics of relevant information, and to update the relevant information of the annual report to the corresponding drug variety files and drug safety credit files in a timely manner
.
The review, inspection, verification, monitoring and evaluation and other specialized drug technical institutions established or designated by the State Drug Administration shall inquire and use the information in the annual drug report according to their duties
.
The Marketing Authorization Holder is the Responsible Body of the Annual Report The Administrative Regulations emphasize that the Drug Marketing Authorization Holder is the responsible body of the annual report and is responsible for its authenticity and accuracy
.
If the holder is an overseas enterprise, the domestic enterprise legal person designated by it shall perform its annual reporting obligations
.
The holder shall write the annual report according to the annual report template.
In principle, one holder shall write one annual report every year
.
The information in the annual drug report shall be true, accurate, complete and traceable, and comply with the requirements of laws, regulations and relevant regulations
.
The annual report shall be approved by the legal representative of the enterprise or the person in charge of the enterprise (or its authorised person in writing), and before April 30 each year, the report information of the previous year shall be reported through the drug annual report system
.
Online filing + information sharing The drug annual report collection module is divided into the enterprise side and the regulatory side
.
The information collected by the enterprise includes two aspects: the public part and the product part
.
The public part includes information on the holder, an overview of quality management, the construction and operation of the pharmacovigilance system, acceptance of overseas commissioned processing, and acceptance of inspections by overseas regulatory agencies; the product part includes product specific information, production and sales, post-marketing research and change management, risk management,
etc.
In order to facilitate enterprises to fill in the report, the basic information of enterprises, basic product information, etc.
can be automatically brought out by the drug annual report collection module in the drug business application system; the approval document number involved in the post-marketing change management, the approval type change, and the record type change.
The record number and related information can be queried through the drug business application system (enterprise side)
.
If the holder fails to submit the annual report as required, in accordance with the provisions of Article 127 of the Drug Administration Law, it shall be ordered to make corrections within a time limit and given a warning; if it fails to make corrections within the time limit, a fine of not less than 100,000 yuan but not more than 500,000 yuan shall be imposed.
fine
.