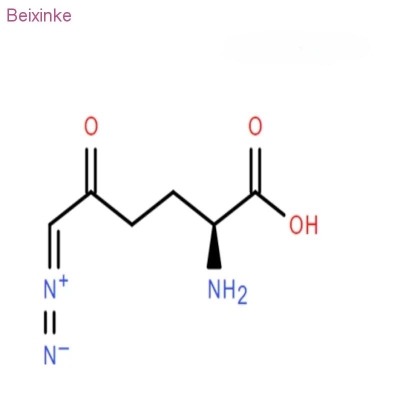
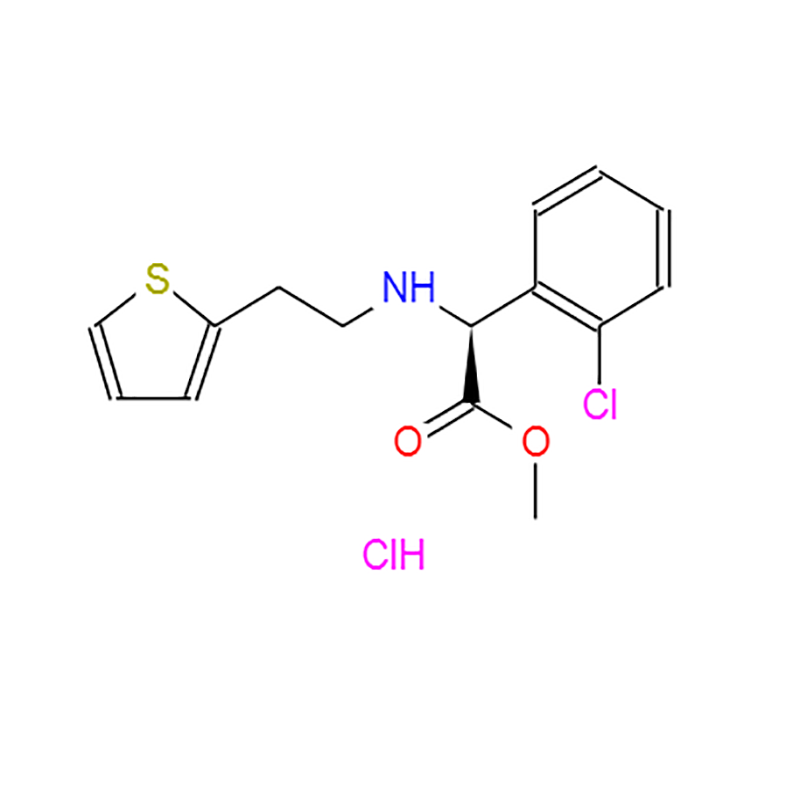
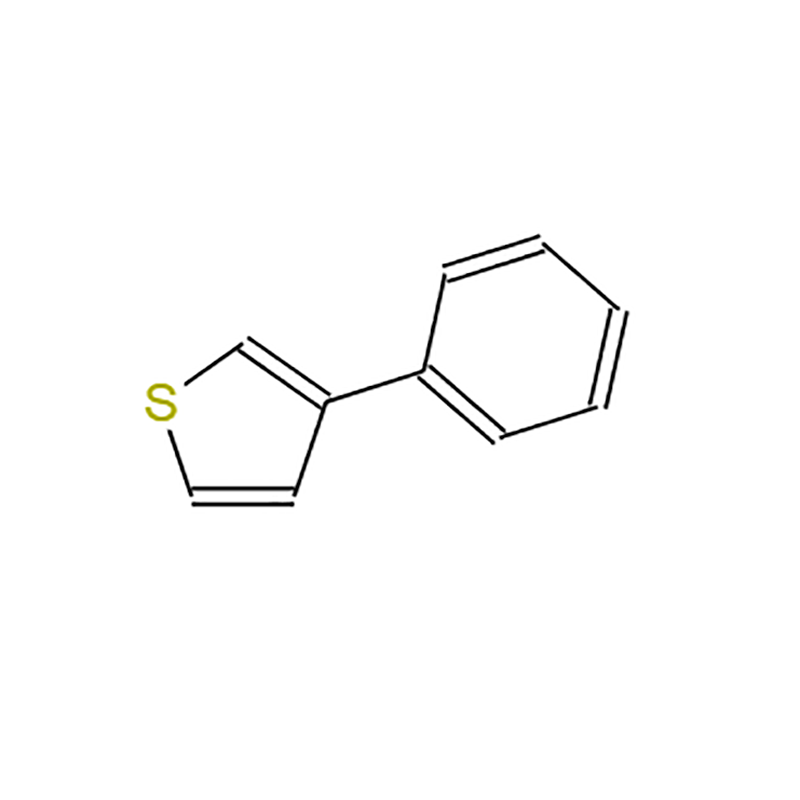
-
Categories
-
Pharmaceutical Intermediates
-
Active Pharmaceutical Ingredients
-
Food Additives
- Industrial Coatings
- Agrochemicals
- Dyes and Pigments
- Surfactant
- Flavors and Fragrances
- Chemical Reagents
- Catalyst and Auxiliary
- Natural Products
- Inorganic Chemistry
-
Organic Chemistry
-
Biochemical Engineering
- Analytical Chemistry
-
Cosmetic Ingredient
- Water Treatment Chemical
-
Pharmaceutical Intermediates
Promotion
ECHEMI Mall
Wholesale
Weekly Price
Exhibition
News
-
Trade Service
The field of ophthalmology is making waves! Recently, Kanghong Pharmaceutical's Class 1 biological drug KH631 ophthalmic injection was approved for clinical trials, and Lifang Pharmaceutical's pyrilaster potassium eye drops were the first to be produced with a new registration classification, and it was the first in China to pass the evaluation
.
According to data from Minai.
com, the sales of terminal ophthalmic drugs in China's public medical institutions in 2022H1 exceeded 6 billion yuan, and the products TOP10 ranibizumab, compacept and aflibercept ranked in the top three, and 4 domestic brands of the brand TOP10 were on the list
.
At present, 14 varieties of ophthalmic drugs have been evaluated, and the eighth batch of dry eye drugs is being prepared
.
.
According to data from Minai.
com, the sales of terminal ophthalmic drugs in China's public medical institutions in 2022H1 exceeded 6 billion yuan, and the products TOP10 ranibizumab, compacept and aflibercept ranked in the top three, and 4 domestic brands of the brand TOP10 were on the list
.
At present, 14 varieties of ophthalmic drugs have been evaluated, and the eighth batch of dry eye drugs is being prepared
.
Ophthalmic medication TOP10! The 3 major products rose by more than 10%, and 4 domestic brands made the list
According to data from Minai.
com, in 2021, the sales scale of terminal ophthalmic drugs in China's urban public hospitals, county-level public hospitals, urban community centers and township health centers (referred to as China's public medical institutions) exceeded 12 billion yuan, a year-on-year increase of 15.
01%; In the first half of 2022, its sales scale exceeded 6 billion yuan, an increase of 0.
61%
over the same period last year.
com, in 2021, the sales scale of terminal ophthalmic drugs in China's urban public hospitals, county-level public hospitals, urban community centers and township health centers (referred to as China's public medical institutions) exceeded 12 billion yuan, a year-on-year increase of 15.
01%; In the first half of 2022, its sales scale exceeded 6 billion yuan, an increase of 0.
61%
over the same period last year.
Sales of terminal ophthalmic drugs in public medical institutions in China (: million yuan)
Source: Minai.
com Competition Pattern of Drug Terminals in China's Public Medical Institutions
com Competition Pattern of Drug Terminals in China's Public Medical Institutions
Among the TOP10 ophthalmic drug products, the first and second ranibizumab injection and compacept ophthalmic injection both exceeded 700 million yuan in the first half of the year, and the sales of aflibercept intraocular injection solution have soared since its launch, ranking third
for the first time.
for the first time.
2022H1 Top 10 terminal ophthalmic drug products in China's public medical institutions
Source: Minai.
com Competition Pattern of Drug Terminals in China's Public Medical Institutions
com Competition Pattern of Drug Terminals in China's Public Medical Institutions
In terms of sales growth rate, only 4 out of 10 products achieved positive growth
.
Among them, the sales of intraocular injection solution of aflibercept increased by 28.
50%; The sales of polyvinyl alcohol eye drops and digitalis diglycoside eye drops increased by 14.
17% and 13.
97%
respectively.
Among the 6 products that declined, sodium hyaluronate eye drops included in the third batch of centralized procurement fell by 33.
79%.
.
Among them, the sales of intraocular injection solution of aflibercept increased by 28.
50%; The sales of polyvinyl alcohol eye drops and digitalis diglycoside eye drops increased by 14.
17% and 13.
97%
respectively.
Among the 6 products that declined, sodium hyaluronate eye drops included in the third batch of centralized procurement fell by 33.
79%.
Among the TOP10 ophthalmic drug brands, foreign brands such as Novartis, Bayer, and Sangtian Pharmaceutical occupy 6 seats, and there are 4 domestic brands on the list, including Compacept ophthalmic injection from Kanghong Pharmaceutical, chymotrypsin for injection for the first biochemical of Shanghai Medicine, polyvinyl alcohol eye drops from Xindong Biotechnology, and praprofen eye drops from Shandong Haishan Pharmaceutical
.
.
2022H1 Top 10 terminal ophthalmic drug brands in China's public medical institutions
Source: Minai.
com Competition Pattern of Drug Terminals in China's Public Medical Institutions
com Competition Pattern of Drug Terminals in China's Public Medical Institutions
The sales of the three foreign brands fell sharply, with Santen's sodium hyaluronate eye drops falling by 34.
60%, levofloxacin eye drops falling by 10.
70%, and Senju Pharmaceutical's praprofen eye drops falling by 10.
15%.
60%, levofloxacin eye drops falling by 10.
70%, and Senju Pharmaceutical's praprofen eye drops falling by 10.
15%.
4 eye drops have been collected, and 1 billion varieties of Waterloo and antibacterial eye drops have risen by 148%
At the beginning of the national centralized procurement, ophthalmic drugs were not included because there were only a few varieties that had been evaluated, and it was not until the third batch of centralized procurement that ophthalmic drugs
appeared.
In the third, fourth and fifth batches of collection, levofloxacin eye drops, olopatadine hydrochloride eye drops, sodium hyaluronate eye drops, and moxifloxacin hydrochloride eye drops were included
successively.
appeared.
In the third, fourth and fifth batches of collection, levofloxacin eye drops, olopatadine hydrochloride eye drops, sodium hyaluronate eye drops, and moxifloxacin hydrochloride eye drops were included
successively.
National centralized procurement of ophthalmic drugs
Levofloxacin eye drops is the third batch of centralized procurement varieties, and the market scale of this variety continued to expand before centralized procurement, with a peak sales of 750 million yuan
in 2019.
The sales of this product fell by 19.
64% and 23.
64% in 2020 and 2021, respectively, and fell by 6% in the first half of 2022 compared with the same period last year, and the market share of Santen Pharmaceutical fell from 96.
96% in 2014 to 56.
98%
in the first half of 2022.
in 2019.
The sales of this product fell by 19.
64% and 23.
64% in 2020 and 2021, respectively, and fell by 6% in the first half of 2022 compared with the same period last year, and the market share of Santen Pharmaceutical fell from 96.
96% in 2014 to 56.
98%
in the first half of 2022.
"Artificial tears" sodium hyaluronate eye drops are one of the main drugs for the treatment of dry eye, and the terminal sales of public medical institutions in China exceeded 1 billion yuan in 2018, and the peak sales was 1.
2 billion yuan
in 2019 。 This product is the fourth batch of centralized procurement varieties, which was implemented around May 2021, and its sales in 2021 and the first half of 2022 fell by 15.
29% and 33.
79% year-on-year, respectively; IMPORT MANUFACTURER URSAPHARM WAS HIT HARD, WITH ITS MARKET SHARE FALLING FROM 42.
25% IN 2018 TO 23.
66% IN THE FIRST HALF OF 2022; The winning bidder, Chengdu Push Pharmaceutical, was approved for marketing in November 2018, and its market share rose to 14.
75% in the first half of 2022, ranking third
in the market.
2 billion yuan
in 2019 。 This product is the fourth batch of centralized procurement varieties, which was implemented around May 2021, and its sales in 2021 and the first half of 2022 fell by 15.
29% and 33.
79% year-on-year, respectively; IMPORT MANUFACTURER URSAPHARM WAS HIT HARD, WITH ITS MARKET SHARE FALLING FROM 42.
25% IN 2018 TO 23.
66% IN THE FIRST HALF OF 2022; The winning bidder, Chengdu Push Pharmaceutical, was approved for marketing in November 2018, and its market share rose to 14.
75% in the first half of 2022, ranking third
in the market.
Sales of sodium hyaluronate eye drops in public medical institutions in China (10,000 yuan)
Source: Minai.
com Competition Pattern of Drug Terminals in China's Public Medical Institutions
com Competition Pattern of Drug Terminals in China's Public Medical Institutions
Olopatadine hydrochloride eye drops are the fourth batch of centralized procurement varieties, and the sales in 2021 and the first half of 2022 decreased by 14.
39% and 37.
45% year-on-year, respectively
.
Novartis' market share fell from 95.
70% in 2014 to 50.
23% in the first half of 2022, and the winning bidders Zhejiang Jianfeng Pharmaceutical and Huinland Pharmaceutical were approved for listing in 2019 and 2020 respectively, and their market share has increased to more than
20% in the first half of 2022.
39% and 37.
45% year-on-year, respectively
.
Novartis' market share fell from 95.
70% in 2014 to 50.
23% in the first half of 2022, and the winning bidders Zhejiang Jianfeng Pharmaceutical and Huinland Pharmaceutical were approved for listing in 2019 and 2020 respectively, and their market share has increased to more than
20% in the first half of 2022.
Moxifloxacin hydrochloride eye drops are the fourth-generation quinolones developed by Novartis, which were first approved for import in China in December 2018, included in Class B of the National Medical Insurance Catalogue at the end of 2019, and included in the fifth batch of centralized procurement in June 2021 and implemented
around October of that year.
In 2020 and the first half of 2022, the sales of moxifloxacin hydrochloride eye drops increased sharply in two waves, increasing by 384.
80% and 147.
95%
respectively.
The winning bidders China Resources Zizhu Pharmaceutical and Kelun Pharmaceutical quickly seized the market share of the original research drugs, with China Resources Zizhu Pharmaceutical occupying nearly 85% of the market share in the first half of 2022, and Kelun Pharmaceutical occupying 10%.
around October of that year.
In 2020 and the first half of 2022, the sales of moxifloxacin hydrochloride eye drops increased sharply in two waves, increasing by 384.
80% and 147.
95%
respectively.
The winning bidders China Resources Zizhu Pharmaceutical and Kelun Pharmaceutical quickly seized the market share of the original research drugs, with China Resources Zizhu Pharmaceutical occupying nearly 85% of the market share in the first half of 2022, and Kelun Pharmaceutical occupying 10%.
Sales of moxifloxacin hydrochloride eye drops in public medical institutions in China (: million yuan)
Source: Minai.
com Competition Pattern of Drug Terminals in China's Public Medical Institutions
com Competition Pattern of Drug Terminals in China's Public Medical Institutions
Yangtze River, Qilu, Xingqi.
.
.
14 varieties reviewed! Blockbuster dry eye medicine preparation and procurement
.
.
14 varieties reviewed! Blockbuster dry eye medicine preparation and procurement
Up to now, 14 varieties of ophthalmic drugs (involving 48 products) have passed or are deemed to have passed the consistency evaluation
.
Among them, Yangzijiang Pharmaceutical has 4 varieties have been evaluated, and Qilu Pharmaceutical and Xingqi Eye Medicine have 3 varieties each evaluated
.
It is worth mentioning that Qilu Pharmaceutical, Xingqi Eye Medicine and a number of eye drops products are under review, and will be deemed to have passed the consistency evaluation
after being approved for production.
.
Among them, Yangzijiang Pharmaceutical has 4 varieties have been evaluated, and Qilu Pharmaceutical and Xingqi Eye Medicine have 3 varieties each evaluated
.
It is worth mentioning that Qilu Pharmaceutical, Xingqi Eye Medicine and a number of eye drops products are under review, and will be deemed to have passed the consistency evaluation
after being approved for production.
Ophthalmic drug evaluation varieties
Source: Minai.
com China Declaration Progress (MED) database
com China Declaration Progress (MED) database
From the perspective of the competitive landscape, there are as many as 13 companies evaluating moxifloxacin hydrochloride eye drops, 10 companies evaluating levofloxacin eye drops, and 6 companies evaluating sodium hyaluronate eye drops, and these three varieties have been included in the national centralized procurement
.
.
8 varieties are exclusively evaluated, including Tafluprost eye drops from Hengrui Pharmaceutical, Brimonidine timolol eye drops from Kelun Pharmaceutical, brimonidine tartrate eye drops from Qilu Pharmaceutical, sodium chloride eye drops from Chengdu Pushi Pharmaceutical, cyclosporine eye drops (II.
) from Xingqi Eye Medicine, etc
.
) from Xingqi Eye Medicine, etc
.
In addition to the varieties that have been included in the national centralized procurement, ophthalmic drugs also have one variety of diquafosso sodium eye drops that meets the national centralized procurement conditions (3 reviews + original research).
The original manufacturer of Diquafosso sodium eye drops is Sangtian Pharmaceutical, the evaluated companies include Hengrui Pharmaceutical, Qilu Pharmaceutical and Yangtze River Pharmaceutical, and the ANDA under review includes 13 enterprises
such as Xingqi Eye Medicine, Zhenzhiming, Chengdu Push Pharmaceutical, Huianland Pharmaceutical, and Zhongsheng Pharmaceutical.
It is worth paying attention
to whether the product will be included in the next batch of collective procurement.
such as Xingqi Eye Medicine, Zhenzhiming, Chengdu Push Pharmaceutical, Huianland Pharmaceutical, and Zhongsheng Pharmaceutical.
It is worth paying attention
to whether the product will be included in the next batch of collective procurement.
Source: Minainet database
Note: Minai.
com's "Drug Terminal Competition Pattern of Public Medical Institutions in China", the statistical scope is: China's urban public hospitals, county-level public hospitals, urban community centers and township health centers, excluding private hospitals, private clinics and village clinics; The above sales are calculated
based on the average retail price of the product at the terminal.
com's "Drug Terminal Competition Pattern of Public Medical Institutions in China", the statistical scope is: China's urban public hospitals, county-level public hospitals, urban community centers and township health centers, excluding private hospitals, private clinics and village clinics; The above sales are calculated
based on the average retail price of the product at the terminal.