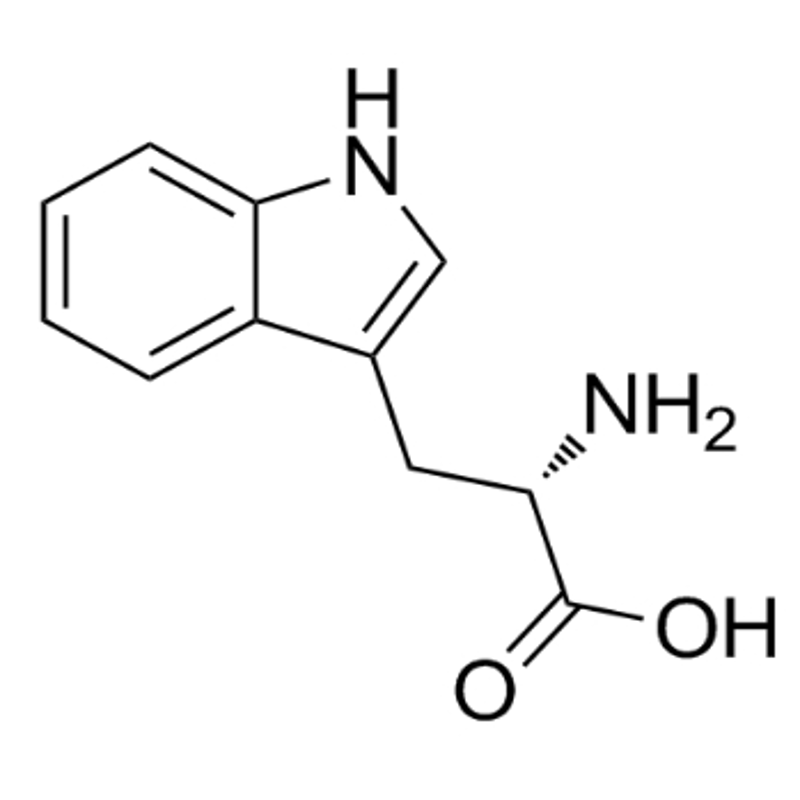
Lysine phosphate calcium: to inform a batch of illegal and serious drug advertising
-
Last Update: 2020-07-03
-
Source: Internet
-
Author: User
Search more information of high quality chemicals, good prices and reliable suppliers, visit
www.echemi.com
Xinhua, Jinan, August 1 (Reporter Wang Yani) Shandong Province Food and Drug Administration recently informed a number of serious illegal drug advertising, to remind patients to pay attention to identification, Shandong Drug Supervision Department law enforcement officials, some of these illegal ads, some without approval, some without the approval of the assertion or guarantee of efficacy, some arbitrarily exaggerate drug indications or functional treatment, some in the mass media to publish prescription drug advertising, some use medical institutions, experts, patients and other names and images as proofdrug regulatory department reported the release of more than 5 illegal serious drug advertising, including: marking Jilin Province, Huinan Huifa Pharmaceutical Co., Ltdproduced "Snow Taian" musk heart-to-brain capsule; Anti-embold pass-through film; mark the brain-heart-in-the-heart capsule produced by Tonghua Shenlong Pharmaceutical Co., Ltd.; Mark the Mingming Quinoa Pill produced by Jilin Correctional Pharmaceutical Group Tonghua City Pharmaceutical Co., Ltd.; Mark the ginseng turtle solid wine produced by Xi'an Detian Pharmaceutical Co., Ltd.; mark the YirenKang capsule produced by Changchun 39 Biopharmaceutical Co., Ltd.; mark the Hengo bone wound healing agent produced by Yunnan Cress Natural Pharmaceutical Stakes, and mark the oral milk of the crow's bile oil produced by Shenyang Oasis Pharmaceutical Co., Ltd; marking the tianma honey cyclobacteria and stretching pills produced by Jilin Jichun Pharmaceutical Co., Ltd.; marking the lysine hydrophosphate calcium particles produced by Bethune Medical University; marking the nine-flavored kidney capsules produced by Liuhe Three Chinese Medicine Co., Ltdin Jilin Province; marking the heart-schumotic capsules produced by Jilin Herbs Pharmaceutical Co., Ltd.; and marking the Shu-Jian waist pills produced by Chen Liji Pharmaceutical Co., LtdXinhua, Jinan, August 1 (Reporter Wang Yani) Shandong Province Food and Drug Administration recently informed a number of serious illegal drug advertising, to remind patients to pay attention to identification, Shandong Drug Supervision Department law enforcement officials, some of these illegal ads, some without approval, some without the approval of the assertion or guarantee of efficacy, some arbitrarily exaggerate drug indications or functional treatment, some in the mass media to publish prescription drug advertising, some use medical institutions, experts, patients and other names and images as proofdrug regulatory department reported the release of more than 5 illegal serious drug advertising, including: marking Jilin Province, Huinan Huifa Pharmaceutical Co., Ltdproduced "Snow Taian" musk heart-to-brain capsule; Anti-embold pass-through film; mark the brain-heart-in-the-heart capsule produced by Tonghua Shenlong Pharmaceutical Co., Ltd.; Mark the Mingming Quinoa Pill produced by Jilin Correctional Pharmaceutical Group Tonghua City Pharmaceutical Co., Ltd.; Mark the ginseng turtle solid wine produced by Xi'an Detian Pharmaceutical Co., Ltd.; mark the YirenKang capsule produced by Changchun 39 Biopharmaceutical Co., Ltd.; mark the Hengo bone wound healing agent produced by Yunnan Cress Natural Pharmaceutical Stakes, and mark the oral milk of the crow's bile oil produced by Shenyang Oasis Pharmaceutical Co., Ltd; marking the tianma honey cyclobacteria and stretching pills produced by Jilin Jichun Pharmaceutical Co., Ltd.; marking the lysine hydrophosphate calcium particles produced by Bethune Medical University; marking the nine-flavored kidney capsules produced by Liuhe Three Chinese Medicine Co., Ltdin Jilin Province; marking the heart-schumotic capsules produced by Jilin Herbs Pharmaceutical Co., Ltd.; and marking the Shu-Jian waist pills produced by Chen Liji Pharmaceutical Co., Ltd(name)
This article is an English version of an article which is originally in the Chinese language on echemi.com and is provided for information purposes only.
This website makes no representation or warranty of any kind, either expressed or implied, as to the accuracy, completeness ownership or reliability of
the article or any translations thereof. If you have any concerns or complaints relating to the article, please send an email, providing a detailed
description of the concern or complaint, to
service@echemi.com. A staff member will contact you within 5 working days. Once verified, infringing content
will be removed immediately.