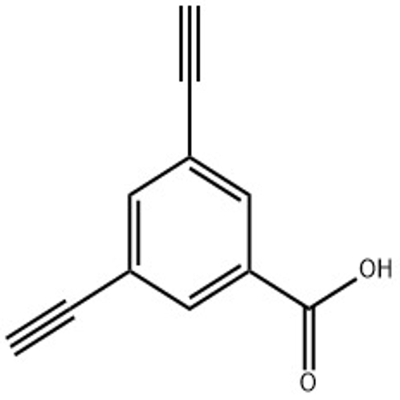
-
Categories
-
Pharmaceutical Intermediates
-
Active Pharmaceutical Ingredients
-
Food Additives
- Industrial Coatings
- Agrochemicals
- Dyes and Pigments
- Surfactant
- Flavors and Fragrances
- Chemical Reagents
- Catalyst and Auxiliary
- Natural Products
- Inorganic Chemistry
-
Organic Chemistry
-
Biochemical Engineering
- Analytical Chemistry
-
Cosmetic Ingredient
- Water Treatment Chemical
-
Pharmaceutical Intermediates
Promotion
ECHEMI Mall
Wholesale
Weekly Price
Exhibition
News
-
Trade Service
Medical Network, August 27 News On August 25, the Gansu Provincial Food and Drug Administration issued a notice on the issuance of several measures to promote the inheritance and innovation of traditional Chinese medicine, formulating 15 relevant measures
.
The original text is as follows:Notice of the Gansu Provincial Drug Administration on Printing and Distributing Several Measures to Promote the Inheritance, Innovation and Development of Traditional Chinese MedicineGanyao Jianfa [2021 ] No.
124Market Supervision Administration of each city and state, relevant departments of provincial bureaus, and directly affiliated institutions: For in-depth implementation Implementing the "Opinions of the Central Committee of the Communist Party of China and the State Council on Promoting the Inheritance and Innovation and Development of Traditional Chinese Medicine", "The Implementation Opinions of the National Medical Products Administration on Promoting the Inheritance and Innovation and Development of Traditional Chinese Medicine", and "Several Measures of the Gansu Provincial Committee of the Communist Party of China on Promoting the Inheritance and Innovation and Development of Traditional Chinese Medicine" "Gansu Province’s Action Plan for Promoting the Construction of a National Comprehensive Experimental Zone for the Development of Traditional Chinese Medicine Industry (2021-2023)" and other relevant requirements, continue to deepen the reform of “delegation, regulation and service”, focus on outstanding problems in the field of traditional Chinese medicine, and adhere to the integrity and innovation of traditional Chinese medicine.
Promote the high-quality development of the Chinese medicine industry, based on the responsibilities of drug supervision and actual work, formulate the following pragmatic measures to promote the inheritance, innovation and development ofChinese medicine :1.
Serve the high-quality development of the Chinese medicine industry1.
Promote the processing of bulk real estate Chinese medicinal materials
.
Give full play to the advantages of Gansu province’s traditional Chinese medicinal materials resources and the national comprehensive experimental zone for the development of traditional Chinese medicine industry, combined with the "Response Letter of the General Department of the State Drug Administration on the Issues Concerning the Procurement of Traditional Chinese Medicine Decoction Pieces Manufacturers’ Procurement of Chinese Medicinal Materials" Facilitate policies, consolidate the results of poverty alleviation, and help rural revitalization.
In the main production areas of bulk real estate Chinese medicinal materials in our province, innovate industrial development models and supervision methods, promote the implementation of bulk real estate Chinese medicinal materials production areas and processing measures, and use the origin-processed Chinese medicinal materials as raw materials.
Provided by the national Chinese herbal medicine production enterprises, continuously strengthen the quality and safety supervision of the whole process from planting to use, improve the quality of Chinese herbal medicines, and promote the high-quality development of the Chinese medicine industry
.
2.
Improve the quality standard system of traditional Chinese medicine
.
Establish and improve the working mechanism for the formulation and revision of relevant standards led by the government, the main body of enterprises, and the participation of the society.
According to the "Chinese Pharmacopoeia", "Standards for Chinese Medicinal Materials in Gansu Province" and "Gansu Province Traditional Chinese Medicine Processing Regulations", gradually improve the quality standards and standards for the production areas of bulk authentic Chinese medicinal materials Processing technology guidelines (standards), standardize the planting and processing quality control of Chinese medicinal materials, and ensure that the product quality meets the standards
.
Accelerate the development of local standards for traditional Chinese medicine formula granules, guide Chinese medicine formula granule manufacturers in the province to formulate quality standards, and promote traditional Chinese medicine formula granule manufacturers to conduct material basic research with universities, scientific research institutions, and medical institutions
.
3.
Expand the field of technical support services
.
Based on the strategic cooperation agreement signed by Gansu Provincial Food and Drug Administration, Lanzhou University, and China Pharmaceutical University, actively carry out regulatory scientific research, and gradually expand the channels of regulatory scientific research through effective measures such as the establishment of the "Gansu Provincial Evidence-based Pharmaceutical Regulatory Scientific Research Institute".
Explore the research and application of Chinese medicine regulatory science and real-world data
.
Give full play to the role of the key laboratory for quality control of Chinese medicinal materials and decoction pieces of the State Food and Drug Administration, carry out basic, critical, forward-looking and strategic regulatory technology research, and provide strong technical support for Chinese medicine regulation and new drug research and development
.
4.
Support industrial upgrading and foreign exchanges
.
Promote the high-end, intelligent and green construction of Chinese medicine production enterprises, strengthen technology integration and process innovation, improve the level of Chinese medicine equipment manufacturing, and accelerate the standardization and modernization of Chinese medicine production processes and procedures
.
With the help of the "One Belt One Road" development strategy, support modern Chinese medicine companies to carry out cooperation and exchanges with foreign universities, scientific research institutions, pharmaceutical companies, etc.
, promote the registration of Chinese medicine products overseas, and continue to expand Longyao's brand influence at home and abroad
.
2.
Innovative Chinese herbal medicine production management 5.
Sharing inspection and testing platform
.
Allowing the Chinese medicine decoction pieces production enterprises controlled by the same park or group to share laboratory inspection equipment under the premise of ensuring the complete and effective operation of the drug production quality control system
.
6.
Entrusted inspection is allowed
.
Pieces of Chinese medicine production enterprises for pesticide residues, heavy metals and harmful elements, aflatoxins test items, after the provincial Food and Drug Administration for the record, could be entrusted to a qualified third party inspection and testing organization for product testing of raw materials
.
7.
Simplify the process of adding varieties
.
After the traditional Chinese medicine decoction pieces production enterprise passes the GMP compliance inspection, it adds new production varieties (except toxic decoction pieces) under the original production scope processing method, and it is compatible with the production scale, and adopts report verification and annual report management without filing
.
3.
Optimize the management of preparations in medical institutions 8.
Improve the management of entrusted preparation of preparations in medical institutions
.
Support medical institutions to select pharmaceutical manufacturers with sound quality management systems to entrust the preparation of traditional Chinese medicine preparations.
Medical institutions can apply for entrusted preparation of preparations at the same time when they first apply for registration and filing
.
9.
Optimize the use of traditional Chinese medicine preparations in medical institutions
.
TCM preparations prepared by the leading unit of the medical consortium (third-level public hospitals ) and clinically used safely for more than 2 years in medical institutions can be directly adjusted and used in their compact medical consortium medical institutions
.
The in-hospital preparation transfer party bears the main responsibility for the quality of the traditional Chinese medicine preparations used for the preparation.
The transfer party shall scientifically evaluate the efficacy and safety of the transferred preparations during clinical use, and timely report the monitoring information of adverse drug reactions
.
10.
Simplify the submission of application materials
.
The prescription comes from the "Catalogue of Ancient Classic Prescriptions" to declare and record traditional Chinese medicine preparations in medical institutions.
The dosage form (decoction can be made into granules) and preparation methods are consistent with the records in ancient medical records, and the medical institution can provide the safety and effectiveness of traditional Chinese medicine preparations.
The pharmacodynamic research materials and literature materials may be exempted if the materials are used to prove the quality and controllability of the quality
.
4.
Support the innovation of traditional Chinese medicine 11.
Promote the conversion of classic names to new drugs
.
Encourage pharmaceutical production enterprises and medical institutions in the province to dig deeper into local TCM resources, strengthen the research and utilization of classics, encourage hospital preparations to become medicines, patent medicines, and support the registration and production of classic Chinese medicine compound preparations
.
For traditional Chinese medicine compound preparations derived from ancient classic prescriptions in the national published catalogue and without marketed varieties, strengthen the technical guidance and service of Chinese medicine registration, help enterprises to declare to the state as required, and promote the transformation of ancient classic names into new medicines
.
12.
Cultivate large varieties of characteristic Chinese medicines
.
Strengthen the key cultivation of the province's characteristic and advantageous varieties, and promote unique Chinese patent medicines such as Duyiwei Capsules, Yuanhu Zhitong Dropping Pills, Danggui Futongning Dropping Pills, Xuanfei Zhishou Mixture, Cistanche Laxative Oral Liquid, Zhenqi Fuzheng series products, etc.
Sub-development, by optimizing production processes, improving quality standards, and clarifying the material basis for drug efficacy, and enhancing the quality, scientific and technological value, clinical value and market competitiveness of Chinese patent medicines
.
5.
Create a fair and orderly market environment 13.
Effectively strengthen post-market supervision of Chinese medicines
.
Supervise and urge enterprises to strictly implement the quality management standards for drug production and operation, strengthen compliance guidance for production and operation, regularly carry out supervision and inspection and sampling inspections, organize special inspections, severely crack down on various violations of laws and regulations in the production, operation and use links, promote pharmacovigilance management, and continue Promote the effective implementation of corporate responsibilities
.
At the same time, optimize the division of powers, reduce repetitive or unnecessary inspection items, promote the standardization and standardization of supervision and inspection, and create a relaxed innovation environment for the development of the industry on the premise of keeping the bottom line of safety
.
14.
Gradually promote the traceability of Chinese medicine decoction pieces
.
Guide enterprises to actively carry out the construction of a traceability system, encourage enterprises to produce and use small packages of coded Chinese medicine pieces, realize traceability in the whole process of production and circulation of Chinese medicine pieces, and strengthen the self-discipline and binding force of the industry
.
15.
Comprehensively improve the level of risk prevention and control management
.
Supervise traditional Chinese medicine companies to establish a pharmacovigilance and adverse reaction monitoring system, strengthen the monitoring of adverse reactions of traditional Chinese medicine preparations, traditional Chinese medicine decoction pieces, and traditional Chinese medicine formula particles, and organize and evaluate the risk signals found in the monitoring in a timely manner and take risk control measures
.
Gansu Provincial Drug Administration August 24, 2021
.
The original text is as follows:Notice of the Gansu Provincial Drug Administration on Printing and Distributing Several Measures to Promote the Inheritance, Innovation and Development of Traditional Chinese MedicineGanyao Jianfa [2021 ] No.
124Market Supervision Administration of each city and state, relevant departments of provincial bureaus, and directly affiliated institutions: For in-depth implementation Implementing the "Opinions of the Central Committee of the Communist Party of China and the State Council on Promoting the Inheritance and Innovation and Development of Traditional Chinese Medicine", "The Implementation Opinions of the National Medical Products Administration on Promoting the Inheritance and Innovation and Development of Traditional Chinese Medicine", and "Several Measures of the Gansu Provincial Committee of the Communist Party of China on Promoting the Inheritance and Innovation and Development of Traditional Chinese Medicine" "Gansu Province’s Action Plan for Promoting the Construction of a National Comprehensive Experimental Zone for the Development of Traditional Chinese Medicine Industry (2021-2023)" and other relevant requirements, continue to deepen the reform of “delegation, regulation and service”, focus on outstanding problems in the field of traditional Chinese medicine, and adhere to the integrity and innovation of traditional Chinese medicine.
Promote the high-quality development of the Chinese medicine industry, based on the responsibilities of drug supervision and actual work, formulate the following pragmatic measures to promote the inheritance, innovation and development ofChinese medicine :1.
Serve the high-quality development of the Chinese medicine industry1.
Promote the processing of bulk real estate Chinese medicinal materials
.
Give full play to the advantages of Gansu province’s traditional Chinese medicinal materials resources and the national comprehensive experimental zone for the development of traditional Chinese medicine industry, combined with the "Response Letter of the General Department of the State Drug Administration on the Issues Concerning the Procurement of Traditional Chinese Medicine Decoction Pieces Manufacturers’ Procurement of Chinese Medicinal Materials" Facilitate policies, consolidate the results of poverty alleviation, and help rural revitalization.
In the main production areas of bulk real estate Chinese medicinal materials in our province, innovate industrial development models and supervision methods, promote the implementation of bulk real estate Chinese medicinal materials production areas and processing measures, and use the origin-processed Chinese medicinal materials as raw materials.
Provided by the national Chinese herbal medicine production enterprises, continuously strengthen the quality and safety supervision of the whole process from planting to use, improve the quality of Chinese herbal medicines, and promote the high-quality development of the Chinese medicine industry
.
2.
Improve the quality standard system of traditional Chinese medicine
.
Establish and improve the working mechanism for the formulation and revision of relevant standards led by the government, the main body of enterprises, and the participation of the society.
According to the "Chinese Pharmacopoeia", "Standards for Chinese Medicinal Materials in Gansu Province" and "Gansu Province Traditional Chinese Medicine Processing Regulations", gradually improve the quality standards and standards for the production areas of bulk authentic Chinese medicinal materials Processing technology guidelines (standards), standardize the planting and processing quality control of Chinese medicinal materials, and ensure that the product quality meets the standards
.
Accelerate the development of local standards for traditional Chinese medicine formula granules, guide Chinese medicine formula granule manufacturers in the province to formulate quality standards, and promote traditional Chinese medicine formula granule manufacturers to conduct material basic research with universities, scientific research institutions, and medical institutions
.
3.
Expand the field of technical support services
.
Based on the strategic cooperation agreement signed by Gansu Provincial Food and Drug Administration, Lanzhou University, and China Pharmaceutical University, actively carry out regulatory scientific research, and gradually expand the channels of regulatory scientific research through effective measures such as the establishment of the "Gansu Provincial Evidence-based Pharmaceutical Regulatory Scientific Research Institute".
Explore the research and application of Chinese medicine regulatory science and real-world data
.
Give full play to the role of the key laboratory for quality control of Chinese medicinal materials and decoction pieces of the State Food and Drug Administration, carry out basic, critical, forward-looking and strategic regulatory technology research, and provide strong technical support for Chinese medicine regulation and new drug research and development
.
4.
Support industrial upgrading and foreign exchanges
.
Promote the high-end, intelligent and green construction of Chinese medicine production enterprises, strengthen technology integration and process innovation, improve the level of Chinese medicine equipment manufacturing, and accelerate the standardization and modernization of Chinese medicine production processes and procedures
.
With the help of the "One Belt One Road" development strategy, support modern Chinese medicine companies to carry out cooperation and exchanges with foreign universities, scientific research institutions, pharmaceutical companies, etc.
, promote the registration of Chinese medicine products overseas, and continue to expand Longyao's brand influence at home and abroad
.
2.
Innovative Chinese herbal medicine production management 5.
Sharing inspection and testing platform
.
Allowing the Chinese medicine decoction pieces production enterprises controlled by the same park or group to share laboratory inspection equipment under the premise of ensuring the complete and effective operation of the drug production quality control system
.
6.
Entrusted inspection is allowed
.
Pieces of Chinese medicine production enterprises for pesticide residues, heavy metals and harmful elements, aflatoxins test items, after the provincial Food and Drug Administration for the record, could be entrusted to a qualified third party inspection and testing organization for product testing of raw materials
.
7.
Simplify the process of adding varieties
.
After the traditional Chinese medicine decoction pieces production enterprise passes the GMP compliance inspection, it adds new production varieties (except toxic decoction pieces) under the original production scope processing method, and it is compatible with the production scale, and adopts report verification and annual report management without filing
.
3.
Optimize the management of preparations in medical institutions 8.
Improve the management of entrusted preparation of preparations in medical institutions
.
Support medical institutions to select pharmaceutical manufacturers with sound quality management systems to entrust the preparation of traditional Chinese medicine preparations.
Medical institutions can apply for entrusted preparation of preparations at the same time when they first apply for registration and filing
.
9.
Optimize the use of traditional Chinese medicine preparations in medical institutions
.
TCM preparations prepared by the leading unit of the medical consortium (third-level public hospitals ) and clinically used safely for more than 2 years in medical institutions can be directly adjusted and used in their compact medical consortium medical institutions
.
The in-hospital preparation transfer party bears the main responsibility for the quality of the traditional Chinese medicine preparations used for the preparation.
The transfer party shall scientifically evaluate the efficacy and safety of the transferred preparations during clinical use, and timely report the monitoring information of adverse drug reactions
.
10.
Simplify the submission of application materials
.
The prescription comes from the "Catalogue of Ancient Classic Prescriptions" to declare and record traditional Chinese medicine preparations in medical institutions.
The dosage form (decoction can be made into granules) and preparation methods are consistent with the records in ancient medical records, and the medical institution can provide the safety and effectiveness of traditional Chinese medicine preparations.
The pharmacodynamic research materials and literature materials may be exempted if the materials are used to prove the quality and controllability of the quality
.
4.
Support the innovation of traditional Chinese medicine 11.
Promote the conversion of classic names to new drugs
.
Encourage pharmaceutical production enterprises and medical institutions in the province to dig deeper into local TCM resources, strengthen the research and utilization of classics, encourage hospital preparations to become medicines, patent medicines, and support the registration and production of classic Chinese medicine compound preparations
.
For traditional Chinese medicine compound preparations derived from ancient classic prescriptions in the national published catalogue and without marketed varieties, strengthen the technical guidance and service of Chinese medicine registration, help enterprises to declare to the state as required, and promote the transformation of ancient classic names into new medicines
.
12.
Cultivate large varieties of characteristic Chinese medicines
.
Strengthen the key cultivation of the province's characteristic and advantageous varieties, and promote unique Chinese patent medicines such as Duyiwei Capsules, Yuanhu Zhitong Dropping Pills, Danggui Futongning Dropping Pills, Xuanfei Zhishou Mixture, Cistanche Laxative Oral Liquid, Zhenqi Fuzheng series products, etc.
Sub-development, by optimizing production processes, improving quality standards, and clarifying the material basis for drug efficacy, and enhancing the quality, scientific and technological value, clinical value and market competitiveness of Chinese patent medicines
.
5.
Create a fair and orderly market environment 13.
Effectively strengthen post-market supervision of Chinese medicines
.
Supervise and urge enterprises to strictly implement the quality management standards for drug production and operation, strengthen compliance guidance for production and operation, regularly carry out supervision and inspection and sampling inspections, organize special inspections, severely crack down on various violations of laws and regulations in the production, operation and use links, promote pharmacovigilance management, and continue Promote the effective implementation of corporate responsibilities
.
At the same time, optimize the division of powers, reduce repetitive or unnecessary inspection items, promote the standardization and standardization of supervision and inspection, and create a relaxed innovation environment for the development of the industry on the premise of keeping the bottom line of safety
.
14.
Gradually promote the traceability of Chinese medicine decoction pieces
.
Guide enterprises to actively carry out the construction of a traceability system, encourage enterprises to produce and use small packages of coded Chinese medicine pieces, realize traceability in the whole process of production and circulation of Chinese medicine pieces, and strengthen the self-discipline and binding force of the industry
.
15.
Comprehensively improve the level of risk prevention and control management
.
Supervise traditional Chinese medicine companies to establish a pharmacovigilance and adverse reaction monitoring system, strengthen the monitoring of adverse reactions of traditional Chinese medicine preparations, traditional Chinese medicine decoction pieces, and traditional Chinese medicine formula particles, and organize and evaluate the risk signals found in the monitoring in a timely manner and take risk control measures
.
Gansu Provincial Drug Administration August 24, 2021