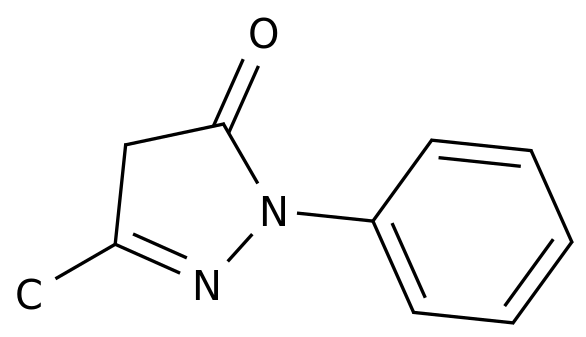
-
Categories
-
Pharmaceutical Intermediates
-
Active Pharmaceutical Ingredients
-
Food Additives
- Industrial Coatings
- Agrochemicals
- Dyes and Pigments
- Surfactant
- Flavors and Fragrances
- Chemical Reagents
- Catalyst and Auxiliary
- Natural Products
- Inorganic Chemistry
-
Organic Chemistry
-
Biochemical Engineering
- Analytical Chemistry
-
Cosmetic Ingredient
- Water Treatment Chemical
-
Pharmaceutical Intermediates
Promotion
ECHEMI Mall
Wholesale
Weekly Price
Exhibition
News
-
Trade Service
Written by Chen Wenqiang | Xi We often can't help but like to eat under certain circumstances.
When we are depressed or encounter inexplicable pressure, we want to relieve our worries with food.
Overeating is often prone to occur at this time.
The pictures of food all over the world broadcast by the media often make us have no resistance to "eating".
Maybe it’s an exquisite food photo at the entrance of a gourmet restaurant, or maybe it’s a shout from our friends.
These are often the key factors in our decision to "eat" or "not eat", rather than our physiology.
Signal of real hunger.
Feeding is a complex motivational behavior that is jointly regulated by homeostatic and non-steady-state mechanisms.
The steady-state mechanisms include metabolism and hormonal signals, while the non-steady-state mechanisms mainly include the environmental factors mentioned in the previous example.
, Such as emotions, cognitive cues.
Clinically, this phenomenon has been described as background-dependent overconsumption, which is manifested as an over-reliance on environmental cues when making "eat" and "not eat" decisions [1].
Understanding the neurobiological mechanisms behind eating or binge eating behaviors caused by non-steady-state mechanisms can help us better intervene in obesity and metabolic diseases.
In-depth study of this mechanism requires the use of rodent models that can effectively simulate clinical practice.
On March 23, 2021, Jeffrey Friedman's team from Rockefeller University in the United States published an online research paper titled Top-down control of conditional overconsumption is mediated by insular cortex Nos1 neurons in Cell Metabolism.
On the basis of neural circuit research methods, it is revealed that a type of specific Nos1-expressing neurons that project from the insular cortex to the central amygdala promotes the neural mechanism of overfeeding, so as to better help us understand the specific environment The neurobiological mechanism behind "bulimia" behavior.
First, the researchers used the specific environmental cues previously reported by the research group to induce binge-eating behavior [2].
Based on Papulov’s conditioning, the researchers trained overnight fasted mice to eat in a specific environment (defined as Ctx+) After 30 minutes, these mice were returned to this environment and found to cause a significant increase in food intake, while placing the mice in other environments (defined as Ctx-) did not find a significant change in food intake (Figure 1 ).
This indicates that mice have established a conditioned association between specific sensory cues, hunger experience, and food availability.
Previous studies have suggested that the insular cortex can encode food-cue associations, and chemical genetic methods that inhibit the activity of the entire insular cortex can inhibit this association [2], suggesting that the insular cortex can be used as a key brain area for studying binge eating behavior.
On this basis, the researchers found that the use of chemical genetic methods to inhibit the insular cortex projection neurons, binge eating behavior can be significantly inhibited.
So, which specific neurons in the insular cortex can regulate this behavior, and what are the downstream targets? Figure 1.
The involvement of the insular cortex in conditioned binge eating behavior is a downstream target for studying the projection neurons of the insular cortex.
The researchers used a variety of tracing techniques (anterograde and retrograde projection) to identify the central amygdala (CeA) as the island The main downstream projection area of leaf cortex.The use of chemical genetic technology to inhibit the insular cortex-central amygdala projection neurons can significantly reduce binge eating behavior.
Subsequent real-time conditional position preference experiments conducted by optogenetic technology suggest that this projection can reduce the reward component of food-cue associations.
In order to study the specific types of insular cortex projection neurons that mediate conditioned binge eating behavior, the researchers used retro-TRAP technology to identify markers of insular cortex-central amygdala projection neurons and found that Nos1 can be used as the Projected molecular logo.
Subsequently, the neurons were specifically labeled by Nos1-Cre mice.
The researchers combined a variety of neuron tracing techniques to identify the neurotransmitter characteristics of the insular cortex-central amygdala projection, and found Nos1 neurons in the insular cortex.
It consists of two specific groups.
One type of glutamatergic Nos1 neurons distributed in the 2/3 layer of the insular cortex can project to the central amygdala, and the other type is traditional GABAergic interneurons.
Using a chemical genetic basis to inhibit the Nos1 neurons in the insular cortex that project to the central amygdala can significantly reduce conditioned binge eating behavior, which proves the necessity of the projection in conditioned binge eating behavior.
Researchers used optical fiber recording technology to detect the activity of Nos1 neurons in the insular cortex during binge eating behavior, and found that the neuron was strongly activated during feeding behavior, further confirming the role of the neuron in binge eating behavior.
Finally, the researchers revealed that Nos1 neurons in the insular cortex regulate the protein kinase delta (PKC-δ) neurons in the central amygdala, and confirmed the existence of this connection through single-synaptic rabies virus tracing technology.
Therefore, these data fully confirm that Nos1 neurons in the insular cortex can project to specific neurons in the central amygdala, thereby inhibiting the PKC-δ neurons in the central amygdala to promote binge eating behavior (Figure 2).
Figure 2.
Nos1 neurons in the insular cortex can inhibit PKC-δ neurons in the central amygdala and promote binge eating behavior.
This article uses a series of neural circuit tracing techniques, chemical genetic manipulation techniques, and neuron activity detection techniques to jointly reveal The glutamatergic Nos1 neurons in the insular cortex can regulate conditioned binge eating behavior through a top-down neural pathway that projects to the central amygdala.
By identifying the molecular characteristics of the loop, it was found that this loop can link environmental cues with significantly increased facility behavior.
This research can better help us understand how sensory cues affect behavior, and further prompt people how to The binge eating behavior that exists in a specific environment, and then understand the environmental factors of obesity. Original link: https://doi.
org/10.
1016/j.
cmet.
2021.
03.
001 Platemaker: 11 References [1] Reynolds, AJ, and Payne, CR (2017).
Understanding the relationship between context dependence and susceptibility to consumption cues: a structured abstract.
In Creating Marketing Magic and Innovative Future Marketing Trends (Springer International Publishing), pp.
719–723.
[2] Stern, SA, Doerig, KR, Azevedo, EP, Stoffel, E.
, and Friedman, JM (2020).
Control of non-homeostatic feeding in sated mice using associative learning of contextual food cues.
Mol.
Psychiatry 25, 666–679.
Instructions for reprinting [Original Articles] BioArt original articles are welcome to be shared by individuals.
Reprinting is permitted, and the copyrights of all published works are owned by BioArt.
BioArt reserves all statutory rights and offenders must be investigated.
When we are depressed or encounter inexplicable pressure, we want to relieve our worries with food.
Overeating is often prone to occur at this time.
The pictures of food all over the world broadcast by the media often make us have no resistance to "eating".
Maybe it’s an exquisite food photo at the entrance of a gourmet restaurant, or maybe it’s a shout from our friends.
These are often the key factors in our decision to "eat" or "not eat", rather than our physiology.
Signal of real hunger.
Feeding is a complex motivational behavior that is jointly regulated by homeostatic and non-steady-state mechanisms.
The steady-state mechanisms include metabolism and hormonal signals, while the non-steady-state mechanisms mainly include the environmental factors mentioned in the previous example.
, Such as emotions, cognitive cues.
Clinically, this phenomenon has been described as background-dependent overconsumption, which is manifested as an over-reliance on environmental cues when making "eat" and "not eat" decisions [1].
Understanding the neurobiological mechanisms behind eating or binge eating behaviors caused by non-steady-state mechanisms can help us better intervene in obesity and metabolic diseases.
In-depth study of this mechanism requires the use of rodent models that can effectively simulate clinical practice.
On March 23, 2021, Jeffrey Friedman's team from Rockefeller University in the United States published an online research paper titled Top-down control of conditional overconsumption is mediated by insular cortex Nos1 neurons in Cell Metabolism.
On the basis of neural circuit research methods, it is revealed that a type of specific Nos1-expressing neurons that project from the insular cortex to the central amygdala promotes the neural mechanism of overfeeding, so as to better help us understand the specific environment The neurobiological mechanism behind "bulimia" behavior.
First, the researchers used the specific environmental cues previously reported by the research group to induce binge-eating behavior [2].
Based on Papulov’s conditioning, the researchers trained overnight fasted mice to eat in a specific environment (defined as Ctx+) After 30 minutes, these mice were returned to this environment and found to cause a significant increase in food intake, while placing the mice in other environments (defined as Ctx-) did not find a significant change in food intake (Figure 1 ).
This indicates that mice have established a conditioned association between specific sensory cues, hunger experience, and food availability.
Previous studies have suggested that the insular cortex can encode food-cue associations, and chemical genetic methods that inhibit the activity of the entire insular cortex can inhibit this association [2], suggesting that the insular cortex can be used as a key brain area for studying binge eating behavior.
On this basis, the researchers found that the use of chemical genetic methods to inhibit the insular cortex projection neurons, binge eating behavior can be significantly inhibited.
So, which specific neurons in the insular cortex can regulate this behavior, and what are the downstream targets? Figure 1.
The involvement of the insular cortex in conditioned binge eating behavior is a downstream target for studying the projection neurons of the insular cortex.
The researchers used a variety of tracing techniques (anterograde and retrograde projection) to identify the central amygdala (CeA) as the island The main downstream projection area of leaf cortex.The use of chemical genetic technology to inhibit the insular cortex-central amygdala projection neurons can significantly reduce binge eating behavior.
Subsequent real-time conditional position preference experiments conducted by optogenetic technology suggest that this projection can reduce the reward component of food-cue associations.
In order to study the specific types of insular cortex projection neurons that mediate conditioned binge eating behavior, the researchers used retro-TRAP technology to identify markers of insular cortex-central amygdala projection neurons and found that Nos1 can be used as the Projected molecular logo.
Subsequently, the neurons were specifically labeled by Nos1-Cre mice.
The researchers combined a variety of neuron tracing techniques to identify the neurotransmitter characteristics of the insular cortex-central amygdala projection, and found Nos1 neurons in the insular cortex.
It consists of two specific groups.
One type of glutamatergic Nos1 neurons distributed in the 2/3 layer of the insular cortex can project to the central amygdala, and the other type is traditional GABAergic interneurons.
Using a chemical genetic basis to inhibit the Nos1 neurons in the insular cortex that project to the central amygdala can significantly reduce conditioned binge eating behavior, which proves the necessity of the projection in conditioned binge eating behavior.
Researchers used optical fiber recording technology to detect the activity of Nos1 neurons in the insular cortex during binge eating behavior, and found that the neuron was strongly activated during feeding behavior, further confirming the role of the neuron in binge eating behavior.
Finally, the researchers revealed that Nos1 neurons in the insular cortex regulate the protein kinase delta (PKC-δ) neurons in the central amygdala, and confirmed the existence of this connection through single-synaptic rabies virus tracing technology.
Therefore, these data fully confirm that Nos1 neurons in the insular cortex can project to specific neurons in the central amygdala, thereby inhibiting the PKC-δ neurons in the central amygdala to promote binge eating behavior (Figure 2).
Figure 2.
Nos1 neurons in the insular cortex can inhibit PKC-δ neurons in the central amygdala and promote binge eating behavior.
This article uses a series of neural circuit tracing techniques, chemical genetic manipulation techniques, and neuron activity detection techniques to jointly reveal The glutamatergic Nos1 neurons in the insular cortex can regulate conditioned binge eating behavior through a top-down neural pathway that projects to the central amygdala.
By identifying the molecular characteristics of the loop, it was found that this loop can link environmental cues with significantly increased facility behavior.
This research can better help us understand how sensory cues affect behavior, and further prompt people how to The binge eating behavior that exists in a specific environment, and then understand the environmental factors of obesity. Original link: https://doi.
org/10.
1016/j.
cmet.
2021.
03.
001 Platemaker: 11 References [1] Reynolds, AJ, and Payne, CR (2017).
Understanding the relationship between context dependence and susceptibility to consumption cues: a structured abstract.
In Creating Marketing Magic and Innovative Future Marketing Trends (Springer International Publishing), pp.
719–723.
[2] Stern, SA, Doerig, KR, Azevedo, EP, Stoffel, E.
, and Friedman, JM (2020).
Control of non-homeostatic feeding in sated mice using associative learning of contextual food cues.
Mol.
Psychiatry 25, 666–679.
Instructions for reprinting [Original Articles] BioArt original articles are welcome to be shared by individuals.
Reprinting is permitted, and the copyrights of all published works are owned by BioArt.
BioArt reserves all statutory rights and offenders must be investigated.