-
Categories
-
Pharmaceutical Intermediates
-
Active Pharmaceutical Ingredients
-
Food Additives
- Industrial Coatings
- Agrochemicals
- Dyes and Pigments
- Surfactant
- Flavors and Fragrances
- Chemical Reagents
- Catalyst and Auxiliary
- Natural Products
- Inorganic Chemistry
-
Organic Chemistry
-
Biochemical Engineering
- Analytical Chemistry
-
Cosmetic Ingredient
- Water Treatment Chemical
-
Pharmaceutical Intermediates
Promotion
ECHEMI Mall
Wholesale
Weekly Price
Exhibition
News
-
Trade Service
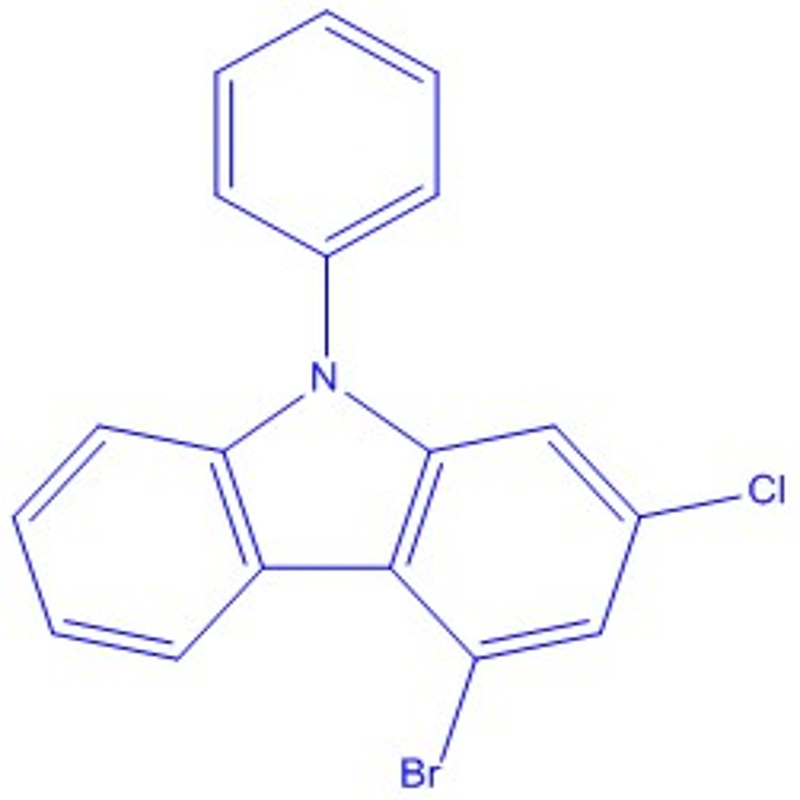
3,6-dibromo-9-phenylcarbazole is an organic compound that is widely used in various applications such as as a hydrotropic solvent, a versatile building block for the synthesis of materials, and as a highly efficient photosensitizer in dye-sensitized solar cells.
The production process of 3,6-dibromo-9-phenylcarbazole involves several steps that need to be carried out in a controlled and efficient manner to ensure the yield of the desired product.
Step 1: Synthesis of N-Phenylbenzamide
The first step in the production process of 3,6-dibromo-9-phenylcarbazole is the synthesis of N-phenylbenzamide.
This is achieved by reacting phenyl acetamide with benzaldehyde in the presence of a weak acid catalyst such as hydrochloric acid.
The reaction is carried out at a temperature of around 150-160 degrees Celsius for several hours.
Step 2: N-Phenylbenzamide Hydrolysis
After the synthesis of N-phenylbenzamide, it is subjected to hydrolysis to remove the benzamide group.
This is achieved by treating the compound with water and a strong acid catalyst such as sulfuric acid.
The reaction is carried out at a temperature of around 80-90 degrees Celsius for several hours.
Step 3: N-Phenylaminobenzene Synthesis
The next step in the production process of 3,6-dibromo-9-phenylcarbazole is the synthesis of N-phenylaminobenzene.
This is achieved by reacting N-phenylbenzamide with ammonia in the presence of a strong acid catalyst such as sulfuric acid.
The reaction is carried out at a temperature of around 100-110 degrees Celsius for several hours.
Step 4: N-Phenylaminobenzene Diazotization
After the synthesis of N-phenylaminobenzene, it is subjected to diazotization to introduce the diazo group.
This is achieved by treating the compound with nitrous acid and hydrochloric acid.
The reaction is carried out at a temperature of around 0-10 degrees Celsius for several hours.
Step 5: 3,6-Dibromo-9-Phenylcarbazole Synthesis
The final step in the production process of 3,6-dibromo-9-phenylcarbazole is the synthesis of the desired compound.
This is achieved by reacting N-phenylaminobenzene with bromine in the presence of a solvent such as carbon tetrachloride.
The reaction is carried out at a temperature of around 50-60 degrees Celsius for several hours.
Quality Control
The quality of the 3,6-dibromo-9-phenylcarbazole produced in the production process is essential for its various applications.
To ensure the quality of the product, several quality control measures are implemented in the production process.
These include:
- Purity Analysis: The purity of the product is analyzed by high-performance liquid chromatography (HPLC) to ensure that it meets the required purity standards.
- Mass Spectrometry Analysis: The mass spectrometry analysis is used to determine the molecular weight of the product and to ensure that it is within the required range.
- Infrared Spectroscopy Analysis: Infrared spectroscopy is used to identify the functional groups in the product and to ensure that they are present in the required amounts.
- Nuclear Magnetic Resonance Spectroscopy Analysis: Nuclear magnetic resonance spectroscopy is used to determine the molecular structure of the product and to ensure that it is consistent